Hyaluronic acid is a natural polymer that can be found in many cosmetic products, both for external use (masks, creams, serums, lotions) and injectable forms. HA stabilized with cross-links is the basis of most modern fillers - preparations designed to eliminate wrinkles and folds, increase volume and change the shape of the lips, correct areas of depression and volumetric modeling of the face and body. Unstabilized hyaluronic acid is included in preparations for mesotherapy (biorevitalization), which, when administered intradermally, help moisturize the skin and stimulate metabolic processes, and also ensure a uniform and prolonged supply and distribution of other active ingredients.
When using preparations of unstabilized hyaluronic acid, allergic reactions, persistent inflammatory process (usually signs of inflammation resolve within several hours or days), and angioedema may be observed as adverse events and complications associated with the drug. These reactions are extremely rare, and a thorough medical history is the best way to prevent them.
Side effects from the use of fillers based on stabilized hyaluronic acid are mainly associated with overcorrection, material migration, the formation of areas with a bluish discoloration of the skin when the drug is applied superficially (Tyndall effect), infection and the formation of granulomas as a manifestation of a foreign body reaction. Cases of local necrosis as a result of embolism/compression of blood vessels and a case of pulmonary embolism after injection in the anogenital area have also been described. All of the above side effects are rare, and practicing doctors have been able to verify that hyaluronic fillers (if we are talking about quality drugs) are safe.
One of the most important clinical advantages of fillers based on stabilized hyaluronic acid is the possibility of its accelerated biodegradation under the action of a specific enzyme – hyaluronidase. Complete elimination of the injected material promotes rapid resolution of adverse reactions. Hyaluronidase medications are used in cases of overcorrection, fibrosis and granulomas formed in response to filler injection, as well as for decompression of blood vessels and prevention of necrosis.
Hyaluronidase
Hyaluronidase is an enzyme (more precisely, a group of enzymes) capable of breaking down hyaluronic acid into oligomers (low molecular weight fragments) (Fig. 1)
. Several types of hyaluronidase have been identified in the human body, both in the cytoplasm of cells and in the extracellular matrix. The coordinated work of these enzymes helps maintain optimal GC balance in connective tissue.
Rice.
1. Hyaluronidase is responsible for the depolymerization of hyaluronic acid, promoting the hydrolysis of the glycosidic bond. Hyaluronidases are synthesized not only in the human body - it is a truly universal enzyme. Most of the functions that hyaluronidases perform are related to their ability to increase tissue permeability by reducing the viscosity of the intercellular matrix. High hyaluronidase activity provides conditions that facilitate the penetration of sperm into the egg. Hyaluronidase is a component of the poisonous secretion of some animals, since by reducing the viscosity of the intercellular matrix of tissues and increasing capillary permeability, it facilitates the spread of toxin from the bite site. This same effect, “seen” in nature, is actively used in medicine when hyaluronidase is introduced into tissues together with drugs, for example, local anesthetics, promoting their distribution in tissues during infiltration anesthesia.
Hyaluronidase in the saliva secretion of leeches contributes to the disruption of the integrity of the vascular wall, resulting in capillary bleeding, and it is blood that leeches feed on. “Digestive” functions are performed by hyaluronidase from the saliva of mammals, as well as secreted by bacteria.
Increased hyaluronidase activity is characteristic of metastatic malignant tumors. Attempts are being made to use drugs that suppress this activity as antitumor agents.
According to the classification compiled by Karl Meyer, hyaluronidases can be divided into types using such characteristics as the source of the enzyme, substrates, conditions, type of reaction catalyzed, and products formed1.
Type I - testicular type hyaluronidases (hyaluronate-endo-β-N-acetylhexosaminidase). As a rule, they are included in pharmaceutical preparations.
Different subtypes of testicular hyaluronidase are found in the testes and sperm of mammals, and fish milk; in lysosomes of cells of different tissues; in some physiological fluids (blood serum, synovial fluid, etc.), as well as in the saliva and salivary glands of mammals, in bee and snake venoms. The final products of hydrolysis are tetrasaccharides.
Testicular hyaluronidase exhibits enzymatic activity in the pH range of 4.0-7.0.
Type II - leech salivary hyaluronidase (hyaluronate-endo-β-glucuronidase).
Type III - microbial hyaluronidases (hyaluronate lyases; eliminating hyaluronate-endo-β-N-acetylhexosaminidase), which are produced by clostridia, bacteria of the genus Pneumococcus, Streptococcus, Staphylococcus
etc. The end products of the enzymatic reaction are hexa-, tetra- and disaccharides.
Hyaluronidase and Longidase®: what are the differences?
Hyaluronidase is the general name for a group of enzymes that break down acidic mucopolysaccharides.
Hyaluronidase is the name given to a whole group of enzymes, so it does not have a single chemical composition. This group includes all substances that can break down acidic mucopolysaccharides, and thereby increase the permeability of connective tissues.
Longidaza® is an original drug, the main active ingredient of which is the enzyme hyaluronidase associated with a high-molecular carrier. Therefore, the medicine acts more efficiently than a pure enzyme with fewer unwanted reactions and at the same time retains its activity for a long time.
Historical background on hyaluronidase
The ability of some enzymes to break down mucopolysaccharides was first discussed in 1928. Then F. Durand-Reynal found out that an extract from bovine testes can increase tissue permeability. Subsequently, this discovery aroused interest, and the topic of enzymes continued to develop actively:
In 1931
the spreading factor, the primary name for a group of enzymes, was isolated from sperm;
In 1936–37
Karl Meyer and his colleagues proved the ability of enzymes to destroy polysaccharide acids, and showed that their action is similar to the action of the autolytic enzyme of bacteria of the genus Streptococcus;
In 1949
Karl Meyer coined the term hyaluronidase to refer to a group of enzymes capable of breaking down acidic mucopolysaccharides.
Subsequently, the action of the enzyme was actively studied by many specialists. Drugs have appeared that increase the bioavailability of drugs by improving tissue permeability.
Preparations with hyaluronidase
The positive properties of a group of enzymes served as the starting point for the creation of a number of drugs. The most common of them is a pure enzyme with the international name hyaluronidase.
Some of them are used in cosmetology, some in medicine. Most of the previous generation drugs are designed to eliminate post-traumatic scars, post-operative sutures, reduce inflammation and increase the bioavailability of other drugs in the form of injections or droppers.
Application of hyaluronidase
In medicine
The use of the enzyme in medicine is more diverse. It is used to treat various diseases accompanied by active growth of connective tissue and inflammation. For example, with the help of the enzyme hyaluronidase, they treat adhesions in the fallopian tubes, affect the source of inflammation in inflammatory diseases of the pelvic organs in men and women, endometriosis, secondary infertility, enhance the effect of antibiotics in many diseases, etc. Indications for use include joint contractures, scleroderma, hematomas and other diseases and pathologies. Hyaluronidase improves tissue trophism and permeability, increases the elasticity of scarred areas, increases joint mobility and facilitates the movement of fluid in the interstitial space, therefore helping to reduce swelling.
Indications for use of hyaluronidase
- Adhesive disease;
- Scar deformities;
- Radiation and trophic ulcers;
- Chronic prostatitis;
- Scleroderma;
- Pulmonary tuberculosis;
- Interstitial cystitis;
- Hematomas;
- Rheumatoid arthritis;
- Peyronie's disease;
- Joint contractures;
- Traumatic lesions of peripheral nerves and plexuses;
- Inflammatory diseases of the pelvic organs in women (adnexitis, endometritis, etc.);
- Arthrosis and more.
Medical preparations of hyaluronidase
In medicine, preparations of testicular hyaluronidase isolated from the testes of cattle are used.
The specific substrate of testicular hyaluronidase is glycosaminoglycans (hyaluronic acid, chondroitin, chondroitin sulfate), which form the basis of the intercellular matrix of connective tissue. As a result of depolymerization under the action of hyaluronidase, glycosaminoglycans lose their viscosity and ability to bind water and metal ions. As a result, the formation of collagen fibers becomes more difficult, the permeability of tissue barriers increases, the movement of fluid in the intercellular space is facilitated, and tissue trophism improves. The clinical consequences of these processes are increased elasticity of connective tissue, reduction of contractures and prevention of their formation, reduction of adhesions, flattening of scars, accelerated resorption of hematomas.
Medical indications for the use of hyaluronidase preparations include burn, traumatic, postoperative scars (keloid, hypertrophic); long-term non-healing wounds and ulcers; stiffness and contractures of joints (after inflammatory processes, injuries), osteoarthritis, ankylosing spondylitis, severe diseases of the lumbar discs; chronic tendovaginitis; scleroderma (skin manifestations); superficial soft tissue hematomas; prevention of the formation of gross scarring of the affected areas of the cornea (in ophthalmology). Hyaluronidase therapy is carried out in preparation for plastic surgery to correct scars.
Another area of application for hyaluronidase drugs is to increase the bioavailability of drugs (antibiotics, cytostatics, antihistamines, radiocontrast compounds, local anesthetics and vaccines) that are administered subcutaneously or intramuscularly.
The widespread use of hyaluronic acid injections in aesthetic medicine has led to the expansion of indications for the use of hyaluronidase: as mentioned above, hyaluronidase is successfully used in the treatment of adverse events and complications of injection plastic surgery, when it is necessary to quickly eliminate the injected drug.
At the same time, we must remember the specificity of this enzyme: there is no point in expecting that hyaluronidase will be able to provide “emergency assistance” when introducing an excessive amount of polylactic acid, collagen, silicone, calcium hydroxyapatite, or in the event of immune reactions to these compounds.
Contraindications for the use of hyaluronidase include individual hypersensitivity, acute inflammatory and infectious diseases, recent hemorrhages, and malignant neoplasms. During pregnancy and breastfeeding, use only for special indications.
Since hyaluronidase improves the absorption of drugs administered subcutaneously or intramuscularly, it should be used with caution in combination with other drugs, as an unpredictable increase in absorption and increased systemic action are possible.
In patients receiving large doses of salicylates, cortisone, ACTH, estrogens or antihistamines, the effectiveness of hyaluronidase may be reduced.
Hyaluronidase is usually available in lyophilized form. The activity of the drug is indicated in international units (IU). Before use, hyaluronidase powder is diluted with sterile saline solution. The preparations do not contain preservatives, therefore they can be used immediately after dilution.
When administered intradermally, hyaluronidase acts for 48 hours.
Allergy to hyaluronidase
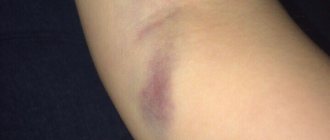
Both at the time of injection and for some time after it, pain may be noted at the site of hyaluronidase injection (therefore, solutions of local anesthetics are sometimes used to dilute hyaluronidase) (Fig. 2)
. 25% of patients experience local reactions in the form of skin hyperemia and swelling2. These reactions resolve spontaneously within 48–72 hours.
Rice.
2. Injection of hyaluronidase: marking the injection area (A), injection of the drug (B). In case of overdose, chills, nausea, vomiting, dizziness, tachycardia, decreased blood pressure, and in extremely rare cases, ventricular fibrillation are observed. In aesthetic medicine, low doses of the drug are used to prevent overdose.
Do not forget that hyaluronidase is a protein drug and therefore has antigenic properties.
A case of anaphylaxis following epidural administration of hyaluronidase has been described3. Immediate type (type I) reactions manifest primarily as swelling, rash, pruritus, pain, respiratory depression, nausea, vomiting, and hypotension. As a rule, such symptoms develop after intravascular administration, and have been noted after injections of hyaluronidase and a chemotherapy drug in the treatment of oncological pathology. Clinical symptoms of allergic reactions are effectively treated with injections of corticosteroids, epinephrine and antihistamines. If symptoms of hypotension occur, vasoconstrictor medications should be used immediately.
After repeated intradermal injection of hyaluronidase, a transient delayed allergic reaction (within 24 hours) may occur, and this is a fairly common occurrence. This reaction is manifested by the formation of large, erythematous, edematous papules or blisters, accompanied by itching, but spontaneously resolving within a few days even without treatment. Local application of corticosteroid ointment in the active phase of the process promotes faster recovery. In such cases, repeated injections of hyaluronic acid should be avoided.
In most patients, hypersensitivity develops after at least one previous injection, which predisposes to the development of sensitization and the appearance of clinical symptoms with subsequent injections. However, there is a possibility that any type of allergic reaction may occur during the first injection, which is why an allergy test is required before any hyaluronidase injection.
Intradermal tests, which are accurate and sensitive, play an important role in assessing the possible risk of developing an allergic reaction to hyaluronidase. Carrying out such a test is not difficult: 1500 IU (standard dosage of the enzyme in foreign preparations. - Editor's note) of hyaluronidase is dissolved in 8-10 ml of physiological solution. Then 0.1 ml of solution (in Russian recommendations - 0.02 ml of solution. - Editor's note
) is administered intradermally in the forearm area. The patient is under the supervision of a doctor for an hour. Any reaction (itching, swelling, redness) at the site of hyaluronic acid injection indicates that the patient is contraindicated for treatment with hyaluronidase due to individual hypersensitivity.
Let us analyze the clinical experience of using hyaluronidase in aesthetic medicine.
Incorrect administration of hyaluronic acid – when and who to contact?
Below is a list of the most alarming symptoms that - if they occur after the injection of cross-linked hyaluronic acid - should prompt the patient to immediately seek help from a specialist experienced in treating complications in the field of aesthetic medicine:
- pain that is not relieved by over-the-counter pain medications;
- increased swelling of the face;
- dyspnea (shortness of breath);
- whitening, decrease or increase in skin temperature over the injection site of hyaluronic acid;
- suspicion of an abscess;
- heat.
Unsatisfactory aesthetic effect, asymmetry, visible lumps and translucency of the drug do not require urgent intervention.
The only correct address where you can eliminate the consequences of complications after using hyaluronic acid is the office of a dermatologist with experience in treating complications in the field of aesthetic medicine.
Hyaluronidase in the treatment of cellulite and fibrosis after liposuction
Clinical experience in this area of one of the authors of the article (F. Depres) totals about 35 years.
Indeed, hyaluronidase has been used for a long time in the treatment of fibrotic cellulite. To do this, 1500 IU of the enzyme was diluted with 8-10 ml of physiological solution and injected deep under the skin (to a depth of at least 1 cm) in the area of fibrosis. It was recommended to inject no more than 3000 IU of hyaluronidase per procedure; the course of treatment included 1-2 procedures, which were carried out once a week. Currently, hyaluronidase preparations are rarely used in the treatment of cellulite, since more effective complex preparations have been developed, including adipolytic ones based on a complex of sodium deoxycholate with phosphatidylcholine.
According to the authors’ experience, early use of hyaluronidase drugs can reduce fibrosis and post-traumatic swelling after liposuction in the breast area (for gynecomastia in men) and in the submental area (“double” chin). These procedures often lead to severe fibrosis, which is reduced very slowly, sometimes over several years. Timely injections of hyaluronidase can significantly reduce or completely eliminate postoperative fibrosis within a short period of time. A single application of 500 IU of hyaluronidase (1500 IU diluted in 9 ml of saline; 3 ml of solution injected in a fan shape) is sufficient to reduce fibrosis in the submental area. At the end of the procedure, a light massage is performed to better distribute the drug. And already during the massage you can notice a decrease in the density of fibrous formations.
Correction of the consequences of excessive administration of stabilized hyaluronic acid
The use of hyaluronidase eliminates the symptoms of overcorrection or technically incorrect injection of fillers based on stabilized hyaluronic acid, for example, with too superficial injection, migration of the drug, injection of a dense drug into thin skin (Fig. 3)
.
Rice.
3. Papules after intradermal injection of stabilized hyaluronic acid in the forehead area. The time interval between the injection of hyaluronic acid and hyaluronidase does not play a special role. However, it is necessary to evaluate the volume of the injected solution and the concentration of hyaluronidase in it to remove exactly the excess of exogenous hyaluronic acid. There are no precise standards on this matter, but it is known that some fillers with stabilized hyaluronic acid are less sensitive to hyaluronidase than others4,5.
In the case of visualized papules (or strands) of superficially injected drugs, one microinjection (literally “at the tip of the needle”) of hyaluronidase (1500 IU of the drug diluted in 4 ml of saline) directly into the center of the papule quickly eliminates the problem.
Quite often we see the consequences of hypercorrection in the periorbital area, when filler is injected to smooth out crow's feet or reduce the severity of the infraorbital groove. At the same time, visually about or bulging of the drug.
A patient came to us with a characteristic clinical picture of hypercorrection in the infraorbital region (Fig. 4 A)
. After a hypersensitivity test, hyaluronidase (1500 IU drug diluted in 4 ml of saline) was injected into three points in the lower eyelid (0.1 ml of solution on each side) using a linear-retrograde technique. The drug was injected into the area where the filler was injected, and quite deeply. After such a procedure, small hematomas and transient swelling may be observed.
After the injection of hyaluronidase, the patient must be under the supervision of the attending physician for an hour, after which an examination is carried out and the result is assessed. As a rule, a control examination is carried out on the 3rd day. In the case described above, we observed a return of the tissue to its original state, unfortunately with wrinkles and depression under the eyes (Fig. 4 B)
. Subsequent reintroduction of stabilized hyaluronic acid may be recommended 2 weeks after hyaluronidase injections. Repeated correction of depression of the infraorbital region should be performed taking into account previous negative experience.
Rice.
4. Visualization of filler injected in excess volume during correction of the infraorbital sulcus (A). Filler based on stabilized hyaluronic acid was introduced 2 years ago. This undesirable phenomenon was corrected using hyaluronidase injections (B). The literature describes cases of overcorrection during lipoplasty6, 7. We only once encountered such a problem when consulting a patient of our colleague. The injection of hyaluronidase quickly corrected the situation without any side effects.
The use of hyaluronidase for the prevention of necrosis
Necrosis that develops as a result of embolism of blood vessels with stabilized hyaluronic acid injected into them is one of the most serious complications of injection plastic surgery. And if earlier we talked about isolated cases of such complications, now, with the rapid growth in popularity of the method, we have to deal with vascular complications more often. And they may not be associated with ignorance of anatomy, but with the atypical location of the vessel in a particular patient.
According to the literature, with early introduction of hyaluronidase into the problem area, it is possible to limit the consequences of ischemia and reduce the risk of necrosis. Unfortunately, injection of hyaluronidase performed after 24 hours of ischemia does not have the desired effect8-10. Kim and colleagues conducted a series of experiments: hyaluronic acid was injected into the ear artery of rabbits; then 4 and 24 hours later, hyaluronidase was injected. A late injection did not cause a decrease in the focus of necrosis, while one carried out after 4 hours made it possible to significantly reduce the area of skin necrosis. Thus, in practice, if symptoms of ischemia appear or are suspected in the area of administration of drugs based on hyaluronic acid, it is necessary to immediately inject hyaluronidase into the problem area10.
What complications after the injection of hyaluronic acid will hyaluronidase help with?
If you contact an experienced cosmetologist-dermatologist in a timely manner, he will be able to help in any situation, except blindness and skin necrosis. Of course, the introduction of hyaluronidase will not give anything if it was not hyaluronic acid that was used, but some other drug of unknown origin. Hyaluronidase acts on any cross-linked hyaluronic acid that is approved for injection in the European Union.
Hyaluronidase
Tyndall effect
Superficial injections of hyaluronic acid can give the skin a different shade than its natural color, both above and around the area where the filler was injected. In the case shown in Fig. 5 A
, the skin in the area of the inner corner of the eye had a clear blue tint.
A filler based on stabilized hyaluronic acid was injected into this area several months earlier, the patient spoke of her satisfaction with the result, with the exception of a change in skin color, which became the motivation for corrective therapy. Hyaluronidase was injected very superficially, ensuring that the drug gets exactly into the implantation area. The enzyme (1500 IU) was diluted in 4 ml of physiological solution and after an intradermal allergy test, 0.2 ml was administered in three lines in a retrograde manner. Thus, the total dose of hyaluronidase was 75 IU on each side. In Fig.
5 B shows the result observed 30 minutes after the therapy.
If necessary, it is possible to repeat the enzymatic hydrolysis procedure, but not earlier than after 2-3 weeks.
If the patient expresses a wish for injection plastic surgery, then such a procedure should be prescribed no earlier than 1-3 weeks after the hyaluronidase injection.
Rice. 5. Tyndall effect (A) and its correction with hyaluronidase injections (B).
Possibilities of using hyaluronidase in the treatment of granulomas
Despite the high biocompatibility of stabilized hyaluronic acid preparations with integumentary tissues, the risk of developing a reaction to a foreign body cannot be completely eliminated. The frequency of such reactions is certainly related to the quality of the drugs.
A delayed-type hypersensitivity reaction (type IV) also develops in the form of granulomas. Granuloma is an accumulation of mononuclear elements, epithelioid and multinucleated giant cells, formed as a result of a chronic proliferative process, as well as as a reaction to exogenous substances and (or) infection. After administration of hyaluronic acid-based preparations, histiocytes and giant cells of foreign bodies predominate in histological samples.
The formation of granulomas often occurs against the background of distinct symptoms of inflammation (redness, swelling, pain, local hyperthermia), which persist for a long period of time. Over time, granulomas (single or multiple) may naturally resolve or require active treatment, the most radical option of which is to remove the drug that caused the immune reaction.
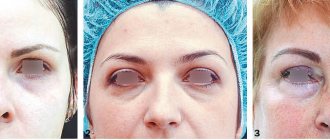
A patient came to us for a consultation who had undergone volumetric correction of the zygomatic area with a filler based on stabilized hyaluronic acid with a good aesthetic result without adverse events or complications. 2 months after injection plastic surgery, she was prescribed a radiofrequency lifting procedure, which involves deep heating of the skin. A week after radiothermal exposure, an inflammatory reaction developed with severe swelling, limited infiltration in the zygomatic region, painful on palpation (Fig. 6 A)
.
Clinical diagnosis - foreign body granuloma (such a diagnosis can only be presumptive. Histological confirmation is required for its verification. - Editor's note
).
After three consecutive injections of hyaluronidase, the patient’s appearance was completely restored, and the symptoms of inflammation were relieved (Fig. 6 B)
. There were no relapses of granuloma formation.
Rice. 6. Granuloma after administration of a drug based on stabilized hyaluronic acid and subsequent local thermal exposure in the same area (A). Result of treatment with three injections of hyaluronidase (B).
What problems require treatment with hyaluronidase?
Most often, patients come to the clinic after unsuccessful lip augmentation in two cases:
- When the drug shines through the mucous membrane because it was not injected deeply enough.
- When unsightly lumps appear on the lips because the drug was administered incorrectly or granulomas have formed.
Also, when introducing hyaluronic acid into different parts of the body, the following complications are possible:
- threatening necrosis of the skin and subcutaneous tissue around the lower lip;
- Tyndall effect or swelling under the eyes caused by too superficial placement of the drug;
- abscesses after increasing the volume of the cheeks;
- early and late allergic reactions.
Lumpiness of lips
Lip necrosis
Swelling of lips
Problems arise due to the administration of a low-quality drug or as a result of the administration of biological products under questionable sanitary conditions.
Many of these patients do not receive care from the esthetician who performed the cross-linked hyaluronic acid procedure because he is not qualified enough to resolve the medical problem in the event of an adverse event. A beauty salon is not a clinic! And a cosmetologist is not a doctor!