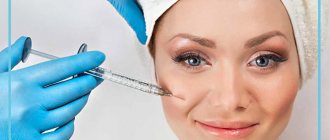
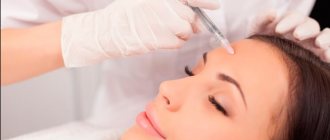
It is prohibited to combine medications with alcohol-containing drinks. But many people want to know exactly which medications do not cause adverse reactions from alcohol. Women who have had beauty injections often ask specialists whether it is possible to combine Dysport and alcohol during treatment.
The instructions for the medicine indicate that it is strictly forbidden to drink alcohol before and after the procedure. It is worth carefully considering the side effects of drinking alcohol during and after treatment.
The effect of alcohol on dysport activity
Drinking alcoholic beverages (including wine and beer) after Dysport injections leads to irreversible processes - when it enters the body, it is quickly absorbed into the blood and distributed to all organs. As a result, it reduces the effectiveness of the procedure, accelerates the removal of toxins from the muscles and causes negative consequences.
Buyanov Sergey Yurievich (Expert Doctor):
Compounds of ethyl alcohol with botulinum toxin enhance the negative effect of the drug, so the likelihood of side effects increases. If without drinking alcohol their manifestations go almost unnoticed, then when drinking ethanol, they are expressed brighter and longer lasting.
Since the brain is first saturated with blood, intoxication occurs instantly - concentration decreases, well-being worsens, weakness, drowsiness and apathy appear. Instead of relaxing the nerve endings, they are activated.
- To get rid of the breakdown products of ethyl alcohol, the liver and kidneys begin to work at maximum capacity. This can lead to overload or disruption of the functioning of the entire body.
- Alcohol provokes dilation of blood vessels and small capillaries, as a result, the rate of diffusion of the drug changes, which causes a rush of blood to the skin.
- Injection wounds bleed heavily and take a long time to heal, which causes excessive swelling for a long time.
- Extensive hematomas occur, which distort facial features.
- Alcohol slows down the removal of fluid from the body and disrupts metabolic processes. This threatens an allergic reaction, poisoning, headache, and nausea.
Women's opinion about the drug
Dysport is a good product that helps restore youth to the face and freshness and smoothness to the skin. Most patients do not think about the consequences of treatment with the drug and go to the salon for the next injection. It is best to first study the reviews of those who have undergone the rejuvenation procedure with this remedy, and then go for a consultation with a doctor.
Negative reviews about the combination of Dysport and alcohol can be found very rarely. Most often, women describe situations where an allergic reaction occurred on the skin. Alcohol-containing products cannot harm humans. But it will be much safer to follow the recommendations of professional cosmetologists and start drinking alcohol only 2 weeks after the injections, since the consequences of combining incompatible products can be dangerous and irreversible.
Drinking alcohol before the procedure
An experienced specialist will refuse to perform the manipulation if the patient had been drinking shortly before treatment. After all, ethanol products are not removed from the blood immediately - they completely leave the body after 3-4 weeks, so you should not drink for at least 15 days before introducing Dysport.
It is strictly forbidden to use it before injections for the purpose of relaxation and pain relief. This is fraught with unpredictable consequences and affects the expression of emotions, which is necessary during the procedure. Special syringes used for injections do not injure the skin or damage tissue. At the request of the patient, the doctor will numb the desired areas with gel.
Dysport®
General recommendations
Recommended doses of Dysport® 500 IU are drug-specific and are not interchangeable with other botulinum toxin A preparations;
Dysport® 500 units should be administered by specialists trained in the administration of treatment.
Focal spasticity of the upper limbs in adults
The maximum total single dose should not exceed 1000 units.
When treating focal spasticity of the upper extremities, Dysport® is diluted to a concentration of 100 U/ml, 200 U/ml or 500 U/ml (see Table 5). The drug is used as intramuscular injection according to the scheme given below.
Dosage during initial and subsequent treatment sessions should be individualized and based on the size, number and location of muscles involved, degree of spasticity, presence of local muscle weakness, patient response to previous treatment and/or history of adverse events during treatment with botulinum toxin complex type A - hemagglutinin.
In clinical studies, doses of 500 IU and 1000 IU were distributed among the muscles involved in the injection session and are indicated in the table below.
The number of injection points depends on the volume of muscles into which injections will be made.
No more than 1 ml of solution can be injected into one point.
Table 1. Dosage of Dysport® for the treatment of focal spasticity of the upper limbs by muscle
| Injected muscles | Recommended doses of Dysport® (IU) |
| Flexor carpi radialis (m. flexor carpi radialis (FCR)) | 100-200 |
| Flexor carpi ulnaris (m. flexor carpi ulnaris (FCU)) | 100-200 |
| Flexor digitorum profundus (m. flexor digitorum profundus (FDP)) | 100-200 |
| Superficial flexor of the fingers (m. flexor digitorum superficialis (FDS)) | 100-200 |
| Flexor pollicis longus (m. flexor pollicis longus) | 100-200 |
| Muscle adductor pollicis (m. adductor pollicis) | 25-50 |
| Brachial muscle (m. brachialis) | 200-400 |
| Brachioradialis muscle (m. brachioradialis) | 100-200 |
| Biceps brachii (m. biceps brachii) | 200-400 |
| Pronator teres (m. pronator teres) | 100-200 |
| Triceps brachii muscle (m. triceps brachii) long head | 150-300 |
| Pectoralis major muscle (m. pectoralis major) | 150-300 |
| Subscapularis muscle (m. subscapularis) | 150-300 |
| Latissimus dorsi (m. latissimus dorsi) | 150-300 |
Although the immediate injection site is determined by palpation, it is recommended to use standard determination techniques such as electromyography, electrical stimulation or ultrasound to select the injection site.
Repeated injections of Dysport® should be carried out after the effect of the previous injection has subsided, but not earlier than after 12 weeks. Most patients in clinical studies received repeat injections after 12 to 16 weeks; however, some patients had a longer effect - up to 20 weeks. The degree and pattern of muscle spasticity at the time of repeat injection may require changes in the dose of Dysport® and the muscles being injected. Clinical improvement can be expected a week after injections of Dysport®.
Elderly patients (≥ 65 years): Clinical experience has not shown differences in response between elderly and younger patients. Elderly patients should be evaluated for tolerance to botulinum toxin type A due to the high incidence of comorbidities and other drug therapies.
Focal spasticity of the lower extremities in adults
During one treatment session, a dose of up to 1500 units can be administered intramuscularly.
When treating focal spasticity of the lower extremities, Dysport® is diluted to a concentration of 100 IU/ml, 200 IU/ml or 500 IU/ml (see Table 5). The drug is used as intramuscular injection according to the scheme given below.
Dosage for initial and subsequent treatment sessions should be individualized and based on the size and number of muscles involved, the degree of spasticity, the presence of local muscle weakness and the patient's response to previous treatment. In clinical studies, doses of 1000 IU and 1500 IU were distributed among the muscles involved in the injection session and are indicated in the table below.
No more than 1 ml of solution can be injected into one point.
Table 2. Dosage of Dysport® for the treatment of focal spasticity of the lower extremities by muscle
| Muscle | Recommended dose range for muscle (IU) | Number of injection points per muscle |
| Main target muscles | ||
| Soleus muscle (m. soleus) | 300-550 units | 2-4 |
| Calf muscle (m. gastrocnemius) | ||
| Medial head | 100-450 units | 1-3 |
| Lateral head | 100-450 units | 1-3 |
| Distal muscles | ||
| Posterior tibialis muscle (m. tibialis posterior) | 100-250 units | 1-3 |
| Flexor digitorum longus (m. flexor digitorum longus) | 50-200 units | 1-2 |
| Flexor digitorum brevis (m. flexor digitorum brevis) | 50-200 units | 1-2 |
| Flexor pollicis longus (m. flexor hallucis longus) | 50-200 units | 1-2 |
| Flexor pollicis brevis (m. flexor hallucis brevis) | 50-100 units | 1-2 |
| Proximal muscles | ||
| Rectus femoris (m. rectus femoris) | 100-400 units | 1-3 |
| Hamstring muscles | 100-400 units | 1-3 |
| Large adductor muscle of the thigh (m. adductor magnus) | 100-300 units | 1-3 |
| Long adductor muscle of the thigh (m. adductor longus) | 50-150 units | 1-2 |
| Short adductor muscle of the thigh (m. adductor brevis) | 50-150 units | 1-2 |
| Gracilis muscle (m. gracilis) | 100-200 units | 1-3 |
| Gluteus maximus muscle (m. gluteus maximus) | 100-400 units | 1-2 |
The degree and pattern of spasticity may change at the time of reinjection, which may require adjustment of the dose of Dysport® and selection of target muscles. Although the immediate injection site is determined by palpation, it is recommended to use standard determination techniques such as electromyography, electrical stimulation or ultrasound to select the injection site.
Repeated injections of Dysport® are carried out every 12-16 weeks or less frequently, based on the return of clinical symptoms, but not earlier than 12 weeks after the previous injection.
Focal spasticity of the upper and lower extremities in adults
If it is necessary to treat spasticity of the upper and lower extremities during one treatment session, the dose of Dysport® for injection into each extremity should be adapted to individual needs and should not exceed a total dose of 1500 units.
Elderly patients (≥ 65 years): Clinical experience has not shown differences in response between elderly and younger patients. Elderly patients should be assessed for tolerance to Dysport® due to the high incidence of concomitant diseases and therapies with other drugs.
Focal spasticity of the lower extremities in children aged 2 years or older
When treating focal spasticity of the lower extremities in children, Dysport® is diluted to obtain the required concentrations in accordance with Table 5. The drug is used for intramuscular injection according to the scheme given below.
Initial and subsequent doses during injection sessions are individualized according to the number, size, location and degree of spasticity of the target muscles, the presence of local muscle weakness, the patient's response to previous treatment and/or previous adverse reactions to botulinum toxin.
The total maximum dose of Dysport administered during an injection session should not exceed 15 units/kg when administered to only one lower limb or 30 units/kg when administered to both lower limbs.
The total dose of Dysport® per treatment session should not exceed 1000 units or 30 units/kg (the lesser of the two).
The total administered dose is distributed between the spastic muscles of the lower limb(s). No more than 0.5 ml of the drug solution can be injected into one point. If it is necessary to inject more than 0.5 ml of solution into one muscle, the total volume of the solution is distributed over several injection points. The table below shows the recommended doses and muscles for administration.
Table 3. Dosage of Dysport® for the treatment of focal spasticity of the lower extremities in children by muscle
| Muscle | Recommended dose range for muscle of one limb (IU/kg body weight) | Number of injection points per muscle/muscle group |
| Distal | ||
| Calf muscle (m. gastrocnemius) | 5-15 units/kg | Up to 4 |
| Soleus muscle (m. soleus) | 4-6 units/kg | Up to 2 |
| Posterior tibialis muscle (m. tibialis posterior) | 3-5 units/kg | Up to 2 |
| Proximal | ||
| Muscles of the back of the thigh (m. semitendinosus, m. semimembranosus, m. biceps femoris) | 5-6 units/kg | Up to 2 |
| Adductor muscles of the thigh (m. adductor longus, m. adductor brevis, m. adductor magnus, m. gracilis) | 3 - 10 units/kg | Up to 2 |
| Total dose | Regardless of whether the injections are carried out only in the distal muscles or only in the proximal ones, or in one treatment session the injections are carried out in both the distal and proximal muscles - the total dose is no more than 15 IU / kg per limb. | |
Although the immediate injection site is determined by palpation, it is recommended to use standard determination techniques such as electromyography, electrical stimulation or ultrasound to select the injection site.
The dosage regimen should be reduced in children:
- with the presence of concomitant diseases associated with problems, in particular with swallowing or breathing;
- those whose target muscles are small;
- who require multi-level injections;
- in those receiving injections under general anesthesia.
In all cases, when choosing a drug dose, an individual assessment of the risk/benefit ratio is required in order to reduce undesirable effects and, in particular, the risk of spread of the toxin remote from the injection site.
Repeated injections of the drug Dysport® are carried out after the effect of the previous injection has decreased, but not earlier than after 12 weeks. Most patients in clinical studies received repeat injections after 16-22 weeks, although some patients had longer-lasting effects of up to 28 weeks. The degree and pattern of spasticity may change at the time of reinjection, which may require adjustment of the dose of Dysport® and selection of target muscles. Clinical improvement usually occurs within 2 weeks after injections of Dysport®.
Focal spasticity of the upper extremities in children aged 2 years or older
When treating focal spasticity of the upper extremities in children, Dysport® is diluted to obtain the required concentrations in accordance with Table 5. The drug is used for intramuscular injection according to the scheme given below.
Dosage for initial and subsequent treatment sessions should be individualized and based on the size, number and location of the muscles involved, the degree of spasticity, the presence of local muscle weakness, the patient's response to previous treatment and/or a history of adverse reactions to botulinum toxin. .
The maximum dose of Dysport® administered during an injection session into one upper limb should not exceed 16 units/kg or 640 units (the lesser of the two). When administering the drug to both upper extremities during an injection session, the maximum dose of Dysport® should not exceed 21 IU/kg or 840 IU (the lesser of the two).
The total administered dose is distributed between the spastic muscles of the upper limb(s). No more than 0.5 ml of the drug solution can be injected into one point. The table below shows the recommended doses and muscles for administration.
Table 4. Dosage of Dysport® for the treatment of focal spasticity of the upper extremities in children by muscle
| Muscle | Recommended dose range for the muscle of one upper limb (IU/kg body weight) | Number of injection points per muscle |
| Brachial muscle (m. brachialis) | 3-6 units/kg | Up to 2 |
| Brachioradialis muscle (m. brachioradialis) | 1.5-3 U/kg | 1 |
| Biceps brachii (m. biceps brachii) | 3-6 units/kg | Up to 2 |
| Pronator teres (m. pronator teres) | 1-2 units/kg | 1 |
| Square pronator (m. pronator quadratus) | 0.5-1 U/kg | 1 |
| Flexor carpi radialis (m. flexor carpi radialis (FCR)) | 2-4 units/kg | Up to 2 |
| Flexor carpi ulnaris (m. flexor carpi ulnaris (FCU)) | 1.5-3 U/kg | 1 |
| Flexor digitorum profundus (m. flexor digitorum profundus (FDP)) | 1-2 units/kg | 1 |
| Superficial flexor of the fingers (m. flexor digitorum superficialis (FDS)) | 1.5-3 U/kg | Up to 4 |
| Flexor pollicis brevis (m. flexor pollicis brevis) | 0.5-1 U/kg | 1 |
| Muscle opponens pollicis (m. opponens pollicis) | 0.5-1 U/kg | 1 |
| Muscle adductor pollicis (m. adductor pollicis) | 0.5-1 U/kg | 1 |
| Total dose | Up to 16 U/kg in one upper limb (and not exceeding 21 U/kg in both upper limbs) | |
Despite the fact that the immediate injection site can be determined based on anatomical landmarks and palpation, it is recommended to additionally use electromyography, electrical stimulation or ultrasound to more accurately position the needle and select the injection site.
Repeated injections of the drug Dysport® are carried out after the effect of the previous injection has decreased, but not earlier than after 16 weeks. Most patients in clinical studies received repeat injections after 16-28 weeks, although some patients had longer-lasting effects of up to 34 weeks. The degree and pattern of spasticity may change at the time of reinjection, which may require adjustment of the dose of Dysport® and selection of target muscles.
Focal spasticity of the upper and lower extremities in children 2 years of age or older
If it is necessary to treat spasticity of the upper and lower extremities in children aged 2 years or older during one treatment session, the dose of Dysport® should not exceed 30 IU/kg or 1000 IU (the lesser of the two).
Repeat injections in the upper and lower extremities should be considered no sooner than 12 to 16 weeks after the previous injection session. The optimal time for retreatment should be determined based on individual progress and response to treatment.
Cervical dystonia in adults
When treating cervical dystonia, Dysport® is diluted to a concentration of 500 U/ml (see Table 5). The drug is used as intramuscular injection according to the scheme given below.
Doses recommended for the treatment of cervical dystonia are used in adult patients of all ages who have normal body weight and satisfactory development of the neck muscles. A reduction in the dose of the drug is possible if the patient is significantly underweight or in elderly people with reduced muscle mass.
The initial total single dose of the drug for the treatment of cervical dystonia is 500 units. This dose is distributed between two or three of the most active muscles of the neck.
For rotational torticollis, the dose of the drug (500 units) is distributed as follows: 350 units into the splenius capitis muscle, ipsilateral to the direction of head rotation, and 150 units into the sternocleidomastoid muscle, contralateral to the rotation.
For laterocollis (head to shoulder tilt), the dose of the drug (500 units) is distributed as follows: 350 units are injected ipsilaterally into the splenius capitis muscle (m. splenius capitis) and 150 units ipsilaterally into the sternocleidomastoid muscle (m. sternocleidomastoideus). In cases involving shoulder elevation due to the trapezius muscle (m. trapezius) or the muscle that lifts the scapula (m. levator scapulae), treatment may be required according to visible muscle hypertrophy or according to electromyographic examination. When injection into three muscles is required, a dose of 500 IU is distributed as follows: 300 IU of the drug is injected into the splenius capitis muscle, 100 IU into the sternocleidomastoid muscle (m. sternocleidomastoideus) and 100 IU into the third muscle (trapezius muscle or levator scapulae muscle).
For retrocollis (tilting the head back), a dose of 500 IU of the drug is distributed as follows: 250 IU in each splenius capitis muscle. Bilateral injections into the splenius capitis muscle may increase the risk of neck muscle weakness.
For the treatment of other forms of cervical dystonia, the use of electromyography (EMG) is of great importance to identify and administer the drug to the most active muscles. EMG should be used to diagnose all complex forms of cervical dystonia or when re-examining patients with no positive dynamics after drug administration, for injections into deep muscles and in patients with excess body weight and difficult-to-palpable neck muscles.
When subsequently prescribing the drug, doses can be adapted in accordance with the effect obtained and the side effects encountered. Recommended total doses range from 250 to 1000 units; the use of higher doses may be accompanied by an increase in the incidence of side effects, in particular dysphagia. The maximum total single dose should not exceed 1000 units.
Injections can be repeated every 16 weeks or as needed, but no more than once every 12 weeks.
The safety and effectiveness of Dysport® in the treatment of cervical dystonia in children has not been confirmed.
Blepharospasm and hemifacial spasm in adults
When treating blepharospasm and hemifacial spasm, Dysport® is diluted to a concentration of 200 U/ml (see Table 5). The drug is used as a subcutaneous injection medially and laterally into the junction between the preseptal and orbital parts of the superior and inferior parts of the orbicularis oculi muscle.
Based on the dose range used in clinical studies, the starting dose of Dysport® for the treatment of blepharospasm is 40 units per eye (based on efficacy/tolerability ratio).
The maximum dose for the treatment of blepharospasm and hemifacial spasm should not exceed 120 units per eye.
Injections in a volume of 0.05 ml (10 units) should be carried out medially and laterally into the connection between the preseptal and orbital parts of the upper (3 and 4) and lower (5 and 6) parts of the circular muscle (m. orbicularis oculi) of each eye. For injections into the upper eyelid, in order to reduce the risk of ptosis, the needle should be directed away from the center so as not to touch the muscle that lifts the upper eyelid (m. levator palpebrae superioris). Below is a diagram showing where the injections will take place.
Injections should be repeated approximately every 12 weeks or as indicated to prevent recurrence of symptoms (but not more frequently than every 12 weeks).
Depending on the severity of the disease, if the effect of the previous injection was not achieved, with each subsequent administration of the drug, the total dose should be increased to:
- 60 units/eye (eg, 0.05 ml (10 units) medially and 0.1 ml (20 units) laterally),
- 80 units/eye: (eg, 0.1 ml (20 units) medially and 0.1 ml (20 units) laterally),
- or up to 120 units/eye: (eg, 0.1 ml (20 units) medially and 0.2 ml (40 units) laterally), above and below each eye, according to the regimen described previously. If spasm affects visual acuity, additional injection points into the frontal muscle (m. frontalis) above the eyebrow (1 and 2) can also be used. Doses of 80 units and 120 units per eye have a longer lasting effect. However, the incidence of adverse reactions, including ptosis, is dose dependent.
In case of unilateral blepharospasm, injections should be limited to the area of the affected eye.
Patients with hemifacial spasm are treated in the same way as patients with unilateral blepharospasm.
Recommended doses are used in adults of any age, including elderly patients.
The safety and effectiveness of Dysport® in the treatment of blepharospasm and hemifacial spasm in children has not been confirmed.
Hyperhidrosis of the axillary region
When treating axillary hyperhidrosis, Dysport® is diluted to a concentration of 200 U/ml (see Table 5). The drug is used intradermally according to the schemes given below.
The recommended starting dose for the treatment of axillary hyperhidrosis is 100 units per axillary area. If the desired effect is not achieved, then a subsequent increase in the dose to 200 IU of Dysport® is possible. The maximum single dose should not exceed 200 units per axillary area.
The area of drug administration is determined by Minor's test.
The test is carried out before treatment and, if necessary, dynamically, at room temperature (22 - 24 ° C) after the patient has rested for 15 minutes.
To carry out the test you need:
- 5% alcohol solution of iodine;
- potato starch;
- marker;
- antiseptic;
- brush;
- gauze napkins.
The patient is in a supine position with his hands under his head. The sweating area is treated with a 5% alcohol solution of iodine and after 1 minute a thin layer of potato starch is applied to this area with a napkin or brush. The test results are assessed after 5 minutes. In the presence of sweating, the treated surface is visually observed to turn blue. The intensity of color (from pale blue to blue-black) correlates with sweating activity. After the test, the area of hyperhidrosis is marked with a marker, then the starch is washed off with alcohol or another antiseptic.
Intradermal injections are carried out at ten points in each axillary region, 10 units of the drug in a volume of 0.05 ml are injected into each point, i.e. 100 units per area. In most cases, the recommended starting dose suppresses sweating for up to 48 weeks. The frequency of repeated injections is determined individually when the initial level of sweating is restored, but not more often than once every 12 weeks. If there is any evidence of a cumulative effect with repeated injections, the timing of repeat injections is determined individually for each patient.
The safety and effectiveness of Dysport® in the treatment of axillary hyperhidrosis in children has not been confirmed.
Temporary improvement in the appearance of moderate to severe facial hyperkinetic folds (expression wrinkles) in adult patients under 65 years of age when the severity of these wrinkles has a significant psychological impact on the patient
When treating facial wrinkles, Dysport® is diluted to a concentration of 200 U/ml (see Table 5). The drug is used according to the schemes given below.
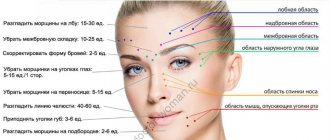
The main area of application of Dysport® for cosmetic correction is the upper half of the face. The lower half of the face and neck are corrected by injecting botulinum toxin much less frequently.
When carrying out injections, it is recommended to use sterile needles of 29-30 G caliber. The total recommended dose for a single injection in all four areas (eyebrow area, forehead area, outer corner of the eye and dorsum of the nose) should not exceed 200 IU.
Eyebrow area
To correct vertical folds in the eyebrow area, injections of the drug are made into the corrugator supercilii muscle (m. corrugator supercilii) 8-10 units per 2-4 points and into the procerus muscle (m. procerus) 5-10 units per 2 points. The total dose ranges from 42 to 100 units.
Forehead area
Elimination of hyperkinetic folds in the forehead area is carried out by injecting the drug into the area of maximum tension of the frontal muscle (m. frontalis). The number of injection points can be arbitrary. All of them should be located 2 cm above the eyebrow line, on the same line or in a V-shape. The optimal total dose of Dysport® in this area is 30-40 units (maximum 90 units) at a rate of 5-15 units per point, the total number of points is 4-6.
Outer corner of the eye area
Correction of folds in the area of the outer corner of the eye (“crow’s feet”) is carried out by subcutaneous injection into points located 1 cm lateral from the outer corner of the eye, at the rate of 5-15 IU of Dysport® per injection point. The number of points is from 2 to 4 for each eye. The maximum recommended total dose on both sides is 120 units.
The frequency of repeated injections depends on the timing of restoration of facial muscle activity. The duration of the effect is 3-4 months.
If an adequate dose of the drug was administered during the first injection, then during the second and subsequent injections the total dose of Dysport® can be reduced by 15-20 IU for the appropriate areas. In this case, it is possible to increase the interval between injections of the drug to 6-9 months. If the initial dose of the drug was insufficient, then with repeated injections it should be increased.
Dorsal area of the nose
To correct wrinkles in the dorsum of the nose, injections are made into the middle of the belly of the nasal muscles. The dose is distributed at 5-10 units to 1-2 points in each muscle.
Insertion points
— — correction of the eyebrow area
▲ — correction of the forehead area
■ — correction of the nasal bridge area
♦ — correction of the outer corner of the eye
The muscle relaxant effect of the drug Dysport® on the facial muscles of the face is clinically manifested on days 2-3 after administration and reaches a maximum on days 14-15. Recommended doses of Dysport® for use in aesthetic medicine do not cause systemic side effects.
Use in children
The safety and effectiveness of botulinum toxin type A therapy for hyperkinetic wrinkles in children under 18 years of age has not been established.
Rules for preparing solution for injection
Remove the protective plastic tamper evident cap from the bottle.
When diluting the drug, do not open the bottle by removing the stopper. Immediately before diluting the contents of the bottle, the central part of the stopper should be treated with alcohol. The lyophilisate is diluted by introducing a regulated volume of 0.9% sodium chloride solution for injection into the bottle by piercing the stopper with a sterile needle of size 23 or 25 (in the case of correction of facial wrinkles, it is recommended to use needles of caliber 29-30 G).
For each indication for use, the required concentrations are specific.
Table 5.
| Received dose in units/ml | Amount of solvent (0.9% sodium chloride solution for injection), ml |
| 500 | 1,0 |
| 200 | 2,5 |
| 100 | 5 |
The resulting solution is a colorless transparent liquid. The diluted drug can be stored for no more than 24 hours at a temperature of 2 °C to 8 °C.
How long should you not drink after
According to most experts, it is enough to give up alcohol for 14 days. This period is optimal for a safe and effective outcome.
There is no ban on the consumption of non-alcoholic beer, kvass, cider and other liquids whose strength does not exceed 3%. But you should not take alcohol-based medications. They may contain a high concentration, which is fraught with the development of complications.
Possible consequences
When drinking alcohol before and after anti-aging injections, the development of complications depends on the individual reaction of the body. According to some experts, there is no influence or it is indirect. But even if this is the case, it is better to play it safe to avoid unpleasant moments.
Symptoms indicating general intoxication:
- headache;
- nausea;
- muscle weakness;
- dry mouth;
- vomit;
- decreased appetite;
- increased body temperature;
- drowsiness;
- deterioration in concentration.
Negative phenomena affecting the aesthetic result:
- drooping or raising of the eyebrows;
- swelling of the eyelids;
- blurred vision;
- facial asymmetry;
- articulation disorder;
- complete immobility of facial expressions;
- blocking lip movement;
- swelling, bruises, bumps.
Alcohol weakens control of actions and inhibits reactions, so there is a possibility of non-compliance with rehabilitation conditions. The patient may simply forget about the rules, which will lead to immediate deterioration of the condition.
Buyanov Sergey Yurievich (Expert Doctor):
Under the influence of alcohol, microcirculation processes are enhanced - more oxygen is supplied to blocked synapses, due to which the blockade by botulinum toxin is weakened. As a result, the effectiveness of the injection drops to zero.