Premature development of mammary glands in girls. Issues of pathogenesis, diagnosis and treatment
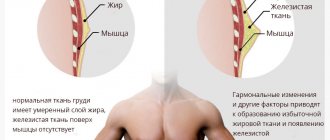
The mammary glands are target organs for the action of various peptide and steroid hormones, therefore they are sensitive to any disturbance in hormonal ratios by changing volume and structure [4,5,14,]. It is assumed that the sensitivity of the mammary glands to hormonal influences appears in all mammals at birth [13], despite the fact that estrogen receptors in individuals of both sexes appear in the epithelium of mammary gland tissue starting from the third trimester of pregnancy, and progesterone receptors - from 2– 3 months after birth [12]. From the moment of birth, the mammary gland is a matrix consisting of fragments of the glandular-ductal complex, immersed in the rudiment of the stromal-fatty complex. At birth, the duct system merges into a common sinus, which opens into a funnel-shaped depression on the skin. Proliferation of mesenchymal cells surrounding the sinus leads to the formation of an inverted nipple, and skin epithelial cells - an areola. Studies of autopsy material from neonatal mammary gland tissue have shown a wide variety of degrees of development of the ductal system, ranging from simple blind-ending structures to well-developed branches with acini. The noted structural variants correspond to the peculiarities of the receptor interaction of epithelial cells of the ducts with estrogens and epidermal growth factor, and the alveolar epithelium of the lobules with prolactin and progesterone of the maternal body. In mammary glands with acini and branching ducts, specialized intra- and interlobular stroma develops [2,9,18]. In 80–90% of newborn girls and a number of boys, the mammary glands increase in size to Ma2–3 by the 3rd–10th days of life, and colostrum begins to separate from the nipples due to the rapid neonatal secretion of pituitary hormones during the period of sexual crisis (PRL, TSH, GH , ACTH) regardless of the structural features of the development of the ductal-alveolar complex [15, 17,18,25]. As the level of pituitary hormones returns to the values of their tonic (basal) secretion, the mammary glands in newborns gradually decrease in size to Ma1 according to Tanner. On palpation, the body of the mammary gland does not exceed 1 cm in diameter and is completely hidden under a non-convex, but sometimes retracted, isola. On echograms, the mammary glands in newborns are represented by oblong-shaped formations of average echogenicity without differentiation of individual structural elements, the dimensions of which are on average 15 mm in length, 6 mm in the anteroposterior direction and 14 mm in width. In this case, the superficial and deep layers of the superficial fascia are clearly visible, which seem to “encompass” the mammary gland [3,8]. In most cases, spontaneous reduction of the mammary glands to their original size occurs within 2–3 weeks. In 1.5–2% of infants, breast enlargement persists until 3–6, and in some cases up to 8–10 months of life. In children aged 1–2 years, only short, small-caliber ductal structures, entwined with a dense stroma of fibroblasts, remain in the mammary glands. A similar structure of the mammary glands remains in children of both sexes until puberty. The resumption of growth and development of the mammary glands in girls occurs at the age of 8–9 years, therefore, until the age of 8, the breast tissue behind the nipple is not palpable, and there is no discharge from the nipples. With premature thelarche, there is an increase in the volume of the mammary glands, usually not exceeding stage 2 of development (Ma2) according to Tanner. The development of the mammary glands in girls with PT is characterized by a more rapid increase in the volume of the left mammary gland. The mammary gland at this stage of development is represented mainly by adipose tissue cells, penetrated by a thin network of stromal elements and a large number of microvessels and neurons surrounding them. That is why increased vascularization and edema, which accompanies the proliferation of ducts, stromal and fatty complexes, causes girls to feel fullness and pain (mastalgia or mastodynia) in the mammary glands. In girls with PT, as a rule, there is no development of the nipples, sexual hair growth and signs of estrogenization of the external and internal genital organs do not appear [19,22]. PT in girls under 8 years of age can occur against the background of persistent follicular cysts, granulosa cell tumors of the ovaries, congenital and/or untreated hypothyroidism (Van Wyck-Grombach syndrome), germ cell tumors producing estrogens, hCG and gonadotropins, as well as with exogenous administration of estrogens and estrogen-like compounds in the form of dosage forms or with food products [2,6,7,17,23,24]. PT occurs in McCune–Albright–Braitsev syndrome (MAS), when premature puberty is caused by uncontrolled activation of estrogen synthesis as a result of a congenital mutation of the receptor protein gene (GSa protein) [11, 20]. In the anamnesis of girls with PT, as a rule, there is no evidence of gross pathology in the antenatal and postnatal periods of life. Physical development corresponds to age. The advance in maturation of the skeletal system does not exceed 1.5–2 years and does not progress further. In some cases, girls with PT have episodic surges in the secretion of FSH and estradiol against the background of pre-pubertal LH levels. Instability of gonadotropic regulation can lead to progression of sexual development in 10% of patients [2,6,19,22]. In girls with isolated PT, in 60–70% of cases, follicles are found in the ovaries, sometimes reaching sizes of 0.5–1.5 cm in diameter. In the hormonal status of children, deviations from the normative indicators for age of LH and FSH are most often absent. When tested with GnRH, girls with premature thelarche are characterized by an increased level of FSH response compared to healthy peers [6,10]. The LH response is prepubertal. Premature thelarche is not accompanied by acceleration of physical development; bone age, as a rule, corresponds to passport age. In girls with PT, spontaneous regression of the mammary glands is possible within 1 year from the moment of their enlargement and further sexual development in accordance with age standards. According to Yu.A. Gurkin, out of 106 girls with isolated PT during further observation, 71 showed a transition to normal puberty, 22 had fibrocystic disease, 11 had a full form of precocious puberty, and 2 girls had delayed puberty [1] . Current international guidelines lack evidence to support the advisability of drug treatment for idiopathic premature thelarche. Annual monitoring and temporary abstinence from vaccinations in girls with premature thelarche are suggested, taking into account the possibility of breast enlargement after vaccination [16,21,24,26,27]. However, in many girls, the increase in volume is accompanied by pain in the mammary glands, causing sleep disturbances, increased excitability and the development of psychopathic reactions. Today in medicine there is an increasingly clear trend towards the development of new, more natural medicines that are no less effective combined with much greater safety of their use. In adolescent girls and women of the reproductive period, herbal remedies containing components of Vitex agnus castus extracts are widely used for mastalgia. According to pharmacological and medical studies, the fruits of chasteberry have a unique ability to interact with D2-dopamine receptors. The dopaminergic effect of components having the chemical structure of diterpenes consists in a dose-dependent inhibition of the formation of cAMP by lactotrophs of the anterior pituitary gland and, thereby, inhibition of prolactin synthesis. Prolactin, together with estrogens and progesterone, controls the entire process of mammogenesis and the formation of intraorgan structures. In addition, the herbal medicine based on Vitex agnus castus normalizes the ratio of gonadotropic hormones, primarily reducing the secretion of FSH. Thanks to its complex effect on the hypothalamic-pituitary system, Vitex agnus castus helps eliminate hormonal imbalances, narrow the ducts, reduce the activity of proliferative processes, and reduce the formation of the connective tissue component. Currently in Russia there is a number of herbal remedies containing Vitex agnus castus. Among them, Cyclodinon O and Mastodinon O (Bionorica AG) take a well-deserved place. Pharmaceutical is a leader in the research and production of herbal preparations based on Vitex sacred in Germany. CyclodinoneO is a single preparation containing only Vitex agnus castus, MastodinonO is a complex herbal preparation, which, in addition to chaste vitex, includes homeopathic dilutions of extracts of alpine violet, iris variegated, cohosh, tiger lily, and chilibucha ignatia. MastodinonO and CyclodinonO are a successful achievement of modern phytoengineering - phytoniring, combining the principles of herbal medicine with modern scientific developments. This is why herbal medicines are just as effective as synthetic ones, but have no side effects. Both of these herbal remedies significantly reduce blood flow and, consequently, swelling of the mammary glands, help reduce pain, and reverse the development of degenerative changes in mammary gland tissue. When taking the drugs, patients note an improvement in their well-being, emotional state and the disappearance of discomfort [1,7]. The problem of mastalgia in girls with PT is no less urgent, but no therapeutic measures are provided. At the request and with the informed consent of the parents, 20 little girls received the herbal medicine Cyclodinon O, containing the fruits of the sacred chasteberry, otherwise called chasteberry (Agni casti fructus), in drops for oral use to eliminate discomfort during PT. 100 grams of solution contains 0.192–0.288 g of dry extract of Agnus castus fruits, corresponding to 2.4 g of medicinal plant material. When examining the girls, a detailed analysis of anamnestic data was used, a hormonal examination was carried out to determine the levels of LH, FSH, PRL, TSH, free thyroxine, estradiol, progesterone, testosterone, 17-OP, DHEA-S, including under the conditions of hormonal stimulation tests, ultrasound pelvic organs, thyroid and mammary glands, according to ultrasound of internal organs, MRI of the brain with contrast. All girls underwent EEG and bone age was determined. The criteria for inclusion in the study were the age of the girl under 8 years old, the absence of endocrine (diabetes mellitus, thyroid disease, EDCG) and sub- and decompensated extragenital pathology. Exclusion criteria were age 8 years and older, refusal to take the drug, presence of adverse reactions, malformations, cysts and tumors of the mammary gland. The girls' ages ranged from 2.5 to 6 years. Clinical and anamnestic data indicated the absence of hereditary burden, pathology of the genital and endocrine organs as a cause of PT. At the same time, a comprehensive examination of the central nervous system (EEG, REG and MRI of the brain) revealed functional changes in the central nervous system in the majority of patients (92%). Psycho-neurological abnormalities in the form of increased nervous excitability, hypertonicity and neurocirculatory dystonia were found in 12 girls. No organic diseases or brain tumors were identified. Anthropometric data and echography of the uterus, ovaries and thyroid gland indicated that their size and structure corresponded to age. The biological age according to x-rays of the hands (bone age) of all examined girls corresponded to the calendar age. Visually, the mammary glands were developed without pronounced asymmetry and looked like a cone without nipple elevation and its pigmentation (stage Ma2 according to Tanner), there was no discharge from the nipples. Palpation revealed pronounced engorgement of both mammary glands. More dense tissue was determined directly behind the areola in the form of a truncated cone. Ultrasound examination of the mammary glands revealed oblong-shaped formations of medium echogenicity without differentiation of individual structural elements. The thickness of the breast tissue ranged from 3–4 to 5–7 mm. Hormonal examination revealed that the levels of FSH and PRL exceeded the age level, while the levels of other peptide and steroid hormones were within the normal range. PRL levels ranged from 477.8 to 728 mIU/L. The concentrations of estradiol and testosterone were below the reference values for age standards. The data obtained made it possible to justify the prescription of Cyclodinone to the examined girls with PT. We selected the following dosage of the drug: children under 3 years of age took 5 drops per 15 ml of water, children under 7 years of age took 10 drops per day for 3 months. At the visit at the end of the 3rd month of treatment, 15 girls showed a reduction in the mammary glands to the age-appropriate degree (Ma1 according to Tanner) and no pain in all 20 girls. In 5 girls, the size of the mammary glands decreased, but complete regression was not observed. As it turned out, these girls suffered from ARVI with hyperthermia during treatment for 4–5 days. Hormonal examination indicated normalization of gonadotropic stimulation while maintaining other hormonal parameters at their original values. Ultrasound data from the genital organs indicated no deviations in the size of the uterus and ovaries from age standards. The mammary glands on echograms were determined by a weakly defined layer of tissue behind the areola, the structure corresponding to the stromal fatty component. In 15 girls with regressed mammary glands, the thickness of the stromal glandular layer was 1.5–3 mm, in the remaining 5 young patients it was 4–5 mm. No signs of pathological formations in the mammary glands were detected, which made it possible to continue taking Cyclodinone for another 3 months at the same dose with a positive effect. During the use of Cyclodinone, taste discomfort, undesirable and side reactions from taking an alcohol-containing solution of Cyclodinone were not observed in any case. Thus, the use of Cyclodinone in drops in girls with premature thelarche indicates good tolerability, the absence of adverse reactions and a pronounced therapeutic effect of Vitex sacred fruits to eliminate premature growth and soreness of the mammary glands. References 1. Gurkin Yu.A. Modern view on the treatment of girls and young women suffering from pathology of the mammary glands Scientific and practical journal Medical Department. – 3(7). – vol. 03, pp. 90–97. 2. Dedov I.I., Semicheva T.V., Peterkova V.A. Sexual development of children: norm and pathology. – M., “Color It Studio” – 2002, pp. 1–232. 3. Zabolotskaya N.V., Zabolotsky V.S. Ultrasound mammography (educational atlas). – M.: 2005. 4. Ilyin A.B., Beskrovny S.V. The mammary gland is an organ of the woman’s reproductive system// Journal of Obstetrics and Women’s Diseases.–2000.–vol. XLIX, issue 2 – P.51–53. 5. Yen S.S.K., Jaffe R.B. Internal genital organs: the effect of steroid hormones on target organs. Reproductive endocrinology. Volume 2: translation from English. M.: Medicine. – 1998. –S. 298–301. 6. Clinical recommendations. Obstetrics and gynecology. Issue 2 ed. V.I. Kulakova. M.: 2008.– 560 P. 7. Levenets S.O., Verkhoshanova O.G., Perevozchikov V.V. Prolactin and its correction in girls with advanced thelarche//Women's Health.–2007.–1 (29).–P 134–139. 8. Ozerova O.E. Normal echographic features of the structure of the mammary glands at different age periods, during pregnancy and lactation//f. SonoAce–International.– N9.– 2001.– P. 50–57. 9. Ozerova O.E. Features of age-related echographic anatomy of the mammary gland (clinical lecture) // Problems of reproduction No. 5 – 2005 P.86–91. 10. Standard principles of examination and treatment of children and adolescents with gynecological diseases and disorders of sexual development. Edited by E.V. Uvarova. “Triad-X”, M. 2004., p.135. 11. Bareille P, Azcona C, Stanhope R: Multiple neonatal endocrinopathies in MCCune–Albright syndrome/ J Pediatr Child Health 1999;35: 315–318. 12. Beatrice A. Howard and Barry A. Custerson. Human breast development.//J. of Mammary Gland Biology and Neoplasia.–2000.–Vol.5, No. 2P.119–137. 118 13. Cardiff RD and Wellings SR The comparative pathology of human and mouse mammary glands.// J. Mammary Gland Biol. Neoplasia.–1999.–Vol. 4–P.105–122. 123. 14. Gruber C.J., Walter Tschugguel M.D., Schneeberger C., Ph.D., and Johannes C. Huber, M.D., Ph.D. Production and Action of Estrogens.//New England Journal of Medicine.–2002. Vol 346 No. 5– R.340–352. 145 15. Hiba J., Pozo ED, Genazzani A., Pustella E., Lancranjan I., Sidiropoulus D., and Gunti J. Hormonal mechanism of milk secretion in the newborn.//J. Clin. Endocrinol. Metab.–1977.–Vol.44–P.973–976. 147 16. Kaplowitz PB, Oberfield SE: Reexamination of the age limit for defining when puberty is precocious in girls in the United States: implications for evaluation and treatment. Drug and Therapeutics and Executive Committees of the Lawson Wilkins Pediatric Endocrine Society. Pediatrics 1999, 104(4 Pt 1): 936–41 16. Kelly PA, Bachelot A, Kedzia C, Hennighausen L, Ormandy CJ, Kopchick JJ, and Binart N. The role of prolactin and growth hormone in mammary gland development.// Mol. Cell. Endocrinol.–2002.–Vol.197–P.127–131. 17. Laurence DJ, Monaghan P., and Gusterson BA The development of the normal human breast. //Oxf. Reu.Reprod.Biol.–1991.–Vol.13–P.149–174. instead of 200 18. Low LC, Wang Q: Gonadotropin independent precocious puberty. J Pediatr Endocrinol Metab 1998 Jul-Aug; 11(4): 497–507 19. Mastorakos G, Mitsiades NS, Doufas AG, Koutras DA: Hyperthyroidism in McCune–Albright syndrome with a review of thyroid abnormalities sixty years after the first report. Thyroid 1997 Jun; 7(3): 433–9. 20. Pediatric Endocrinology and Growth JKH Wales et al.–2nd edition, 2003, p.41–85 21. Quigley CA, Pescovitz OH: Premature thelarche and precocious puberty. Curr Ther Endocrinol Metab 1997; 6: 7–13 22. Quigley Ca, Pescovitz OH: Premature Theelarche and Precocious Puberty. Curr the Endocrinol Metab 1997; 6: 7–13 23. R Stanhope, C.Traggiai. Precocious Puberty (Complete, Partial). Sultan C (ED): Pediatric and Adolescent Gynecology. Evidence - Based Clinical Practice. Endocr dev. Basel, Karger, 2004, Vol.7. PP 57–65. 24. Russian J. and Russian IH Development of the Human Mammary Gland. In Neville MC Daniel CW (EBS.) // The Mammary Gland: Development Regulation and Function, Plenum Press, New York.–1987. - P.67–93184 25. SHANKAR RR, PESCOVITZ OH. Precocious Puberty. Adv Endocrinol Metab 1995; 6: 55–89. 26. Williams Textbook of Endocrinology P.reed Larsen et all.– 10th Edition, 2002, P.1170–1187.
Pediatricians:
Avzalova Daria Evgenevna
Pediatrician, neonatologist
Experience: 18 years Reviews: 7
Call to home
Barzenok Tatyana Arsenyevna
Head of the pediatric department, pediatrician of the first category
Experience: 28 years Reviews: 17
Make an appointment Call at home
Belousova Elena Sergeevna
Pediatrician, nephrologist
Experience: 18 years Reviews: 14
Make an appointment Call at home
Bykov Mikhail Viktorovich
Pediatrician of the highest category, ultrasound diagnostics specialist, Candidate of Medical Sciences
Experience: 25 years Reviews: 3
Make an appointment Call at home
Kazakova Liliya Valentinovna
Pediatrician, neonatologist, head of the breastfeeding consultant service
Experience: 28 years Reviews: 31
Make an appointment Call at home
Sedova Maria Sergeevna
Pediatrician, allergist-immunologist
Experience: 20 years Reviews: 30
Make an appointment Call at home
Sergienko Tatyana Yakovlevna
Pediatrician, pediatrician on duty at the pediatric hotline
Experience: 39 years Reviews: 21
Make an appointment