Hyaluronic acid (HA), or hyaluronate, is one of the most important components of the intercellular fluid. It is “responsible” for retaining moisture in tissues, participates in the process of cell division, and ensures the circulation of lymphocytes, blood cells and oxygen in the body.
With age, the synthesis of hyaluronate decreases, which leads to tissue aging, including the skin. Injections of hyaluronic acid into problem areas help compensate for its deficiency and restore the skin to its former youth and elasticity. This method not only provides a significant anti-aging effect, but also allows you to correct facial features, which in general makes contouring a good alternative to plastic surgery.
Thanks to hyaluronic acid injections you can:
- eliminate wrinkles, including vertical folds between the eyebrows, wrinkles on the forehead, lips, eyes, neck and décolleté;
- smooth out nasolabial folds, nasolacrimal and lacrimal grooves;
- carry out lip contouring, that is, correct their shape;
- carry out correction of the earlobe and nose.
History of discovery and terminology
According to the formula, hyaluronic acid refers to glycosaminoglycans, the molecules of which consist of repeating units that do not contain sulfate groups. For the first time, this high-molecular compound was isolated from the vitreous body of cattle. At first, scientists assumed that the substance was characteristic only of mammals. However, in 1937 this was refuted - it was obtained from a liquid medium in which hemolytic streptococcus was cultivated. In 1954, the structural formula of hyaluronic acid was first published in the British general scientific journal Nature.
The commonly used name of the substance is associated with the history of its discovery (English: “hyaloid” - glassy, “uronic acid” - uronic acid). In international chemical terminology, there is also the name “hyaluronan”, which combines the acid and its salts. The chemical formula of hyaluronic acid is: C₂₈H₄₄N₂O₂₃.
Currently, the range of its application is very wide: medicine, cosmetology, pharmacy. Hyaluronic acid is used as a main and auxiliary substance. The properties of the compound discovered in recent years have great prospects for use in the future, so the demand for this biopolymer is constantly growing.
Glycosaminoglycans[edit | edit code]
Formula of chondroitin sulfate, one of the glycosaminoglycans.
Glycosaminoglycans (GAGs) or mucopolysaccharides are unbranched polysaccharide chains built from repeating disaccharide units in which one of the two sugar residues is an amino sugar (N-acetylglucosamine or N-acetylgalactosamine), which in most cases is sulfated. The presence of sulfate or carboxyl groups (or both) on many sugar residues gives glycosaminoglycans a large negative charge. The second sugar is usually an uronic acid (glucuronic or iduronic). There are four main groups of GAGs:
1) Hyaluronate.
2) Chondroitin sulfate and dermatan sulfate.
3) Heparan sulfate and heparin.
4) Keratan sulfate.
GAGs carry a large negative charge, are highly hydrophilic, have a highly elongated conformation, and form gels even at low concentrations. The recruitment of osmotically active cations by GAG causes swelling pressure (matrix turgor), which gives the matrix the ability to resist compressive forces. Because GAGs form porous hydrated gels, they fill most of the available space and provide tissue support while allowing water-soluble molecules to diffuse and cells to migrate.
Structure
The hyaluronic acid formula is a typical anionic polysaccharide. Molecules are connected in long linear chains. Related substances, glycosaminoglycans, have a large number of sulfated groups. This explains the formation of various isomers - compounds that differ in the spatial arrangement of atoms. Their chemical properties also differ. Hyaluronic acid, unlike glycosaminoglycans, is always chemically identical. Its properties do not depend on the methods of production and the type of starting materials.
Hyaluronic acid contains D-glucuronic acid and N-acetyl-D-glycosamine, which are connected by a beta-glycosidic bond and form its disaccharide units (glucopyranose rings with a molecular weight of about 450 Da). Their number in the molecules of this compound can reach 25,000. Due to this, the acid has a high molecular weight (5,000-20,000,000 Da).
The structural formula of the disaccharide fragment of hyaluronic acid is shown in the figure below.
The acid contains hydrophobic and hydrophilic sections, due to which this high-molecular compound in space has the appearance of a twisted ribbon. The combination of several chains forms a ball of loose structure. The ability to bind and retain up to 1000 molecules of water is another feature of the hyaluronic acid formula. The biochemistry of this substance is primarily due to its high hygroscopicity, which ensures tissue saturation with water and maintenance of internal volume.
Chemical properties
Hyaluronic acid has the following characteristic chemical properties:
- formation of a large number of hydrogen bonds;
- creation of an acidic reaction in aqueous solutions due to the presence of a deprotonated carboxyl group;
- formation of soluble salts with alkali metals;
- the formation in an aqueous solution of a strong gel structure (pseudogel) containing a significant amount of moisture (protein complexes often precipitate);
- creation of insoluble complexes with heavy metals and dyes.
Externally, aqueous solutions of the substance resemble the consistency of egg white. The structural formula of hyaluronic acid allows it to take several forms, depending on the ionic environment of the medium:
- left single helix;
- multi-strand flat structures;
- double helix;
- supercoiled structures with a dense molecular network.
The latter form is tertiary and is capable of absorbing a large volume of water, electrolytes, and high molecular weight proteins.
Characteristics of the physical indicators of the formula
The polymer has an amorphous powder form, having a pure white color, finely dispersed composition. Hyaluronic acid has a linear molecular structure with a high degree of asymmetry.
Physical properties include numerous characteristics, the main ones being:
- Molecular weight – 50-8000 kDa. In comparison with the natural compound, whose mass is 1-20 thousand kDa, it is slightly lower. The average mass of molecules in the human body (synovial fluid) is 3-3.5 thousand kDa.
- Active polymer compound. Optical movement of components
= – 70 – – 80º.
- Solubility in pure water;
- Solubility in aqueous compound with NaCl;
- Does not dissolve when combined with organic substances;
- Included in the group of natural polyelectrolytes with a high degree of viscosity;
- Regulating and stabilizing the water balance of skin tissue.
- It is a hydrophilic polymer.
The stabilizing functionality of the compound is unique.
Hyaluronic acid has a high degree of creation and transformation into gels when interacting with water. The volume of liquid significantly exceeds the mass of acid.
A good example is the structure of the eye body. The ratio of acid and aqueous solution in it is 1/98. The gel formed by the acid does not evaporate, firmly holding a huge volume of water.
The physical characteristics studied and identified by scientists have become widely used in medicine and cosmetology.
Differences between hyaluronic acid of different origins
As mentioned above, the structure of this substance is very similar regardless of the source of its preparation. The difference between acids of bacterial and animal origin is the degree of their polymerization. The hyaluronic acid formula obtained from animal sources has a greater length compared to the bacterial form (4,000-6,000 and 10,000-15,000 monomers, respectively).
The solubility in water of these substances is the same and depends mainly on the presence of hydroxyl and salt groups in disaccharide residues. Since the chemical structure of the acid is inherently similar in all living species, this minimizes the risk of negative immunological reactions and rejection when it is introduced into the body of humans and animals.
Role in nature
The main location of hyaluronic acid is the composition of the intercellular (or extracellular) matrix of mammalian tissues. As scientific research shows, it is also found in the capsules of some bacteria - streptococci, staphylococci and other parasitic microorganisms. Synthesis of the compound also occurs in the body of invertebrate animals (protozoa, arthropods, echinoderms, worms).
Scientists suggest that the ability to produce hyaluronic acid in bacteria was formed evolutionarily to increase their virulent properties in the host body. Thanks to its presence, microorganisms can easily penetrate the skin and colonize it. Such parasitic bacteria are capable of neutralizing the host’s immune response and provoke the development of a more active inflammatory process than other strains of microbes.
Hyaluronic acid is produced by proteins that are embedded in the cell wall or membranes of intracellular organelles. The highest concentration of the substance in the human body is observed in the fluid that fills the cavities of the joints, in the umbilical cord, the vitreous body of the eye and the skin.
Application[edit | edit code]
Being a common polysaccharide (albeit a very long one), hyaluronic acid, or hyaluronan, can be produced in the body using ordinary starch, which is also a long chain of sugars called glucose.
Therefore, to increase hyaluronic acid in the body, it is recommended to eat more starchy foods, such as root vegetables or simply potatoes (link 1):
A study conducted in Japan found that people living in Yuzurihara, a Japanese village, had good eyesight, wrinkle-free skin, and better overall health even at age 90. It was found that these people grew and ate starchy root vegetables (actually three varieties of potatoes) that promoted the production of hyaluronic acid.
If you don’t strictly believe that eating a lot of potatoes will give you plump lips and not a belly, here’s another option.
Until recently, the main source of hyaluronic acid production was rooster combs; they are still used today for the production of hyaluronic acid used in orthopedics - for intra-articular injections of hyaluronic acid.
Also, any cartilage is rich in hyaluronic acid: broth from bones, cartilage and joints, or aspic, should be rich in hyaluronic acid.
Eyes are also rich in hyaluronic acid; you can use, for example, whole fish heads. Also, such products consist almost entirely of collagen, another important material for maintaining the elasticity of both the skin and joints and ligaments.
If you are a modern hipster and don’t want to eat old-fashioned grandma’s bone broth, fish soup or jellied meat, you can always buy modern dietary supplements that are made from hyaluronic acid synthesized by bacteria living in any nutrient medium: wheat, soy, etc. .P. The cost of hyaluronic acid synthesized by bacteria approaches the cost of the raw materials used for the bacterial nutrient medium: the cost of soybeans, wheat, and the like. Therefore, this method of obtaining is currently the cheapest.
If you are a crazy lady, you can also go to your cosmetologist and get an injection of hyaluronic acid for 10 - 50 thousand rubles per procedure. At the same time, it is advisable to keep in mind the cost of hyaluronic acid, which is somewhere between the production of starch and the cost of chicken heads.
Also, cunning nutritionists often recommend vitamin C instead of hyaluronic acid, this is due to the fact that hyaluronic acid is a poor skin protector, for example, with a sunburn, the production of hyaluronic acid in the skin completely stops, which indicates that the skin does not need it at that moment, those. that hyaluronic acid is a poor defense for the body. For normal functioning of skin containing large amounts of hyaluronic acid, large doses of retinol and vitamin C are needed, which are known to be strong antioxidants.
Sometimes, instead of hyaluronic acid, nutritionists recommend foods rich in retinol: cod liver, fatty fish or seafood. Which suggests that the main role of hyaluronic acid is to retain water, and the rest is done by other substances: proteins (collagen), fats (omega-3) and vitamins (retinol, vitamin C).
Sometimes nutritionists are more honest: they say that to maintain skin you need retinol, vitamin C, and hyaluronic acid, and not just hyaluronic acid.
Metabolism
The synthesis of hyaluronic acid takes place in the form of enzymatic reactions in 3 stages:
- Glucose-6-phosphate – glucose-1-phosphate (phosphorylated glucose) – UDP-glucose – glucuronic acid.
- Amino sugar – glucosamine-6-phosphate – N-acetylglucosamine-1-phosphate – UDP-N-acetylglucosamine-1-phosphate.
- Glycoside transferase reaction involving the enzyme hyaluronate synthetase.
Approximately 5 g of this substance is produced and broken down in the human body per day. The total amount of acid is about seven thousandths of a percent by weight. In vertebrates, acid synthesis occurs under the influence of 3 types of enzyme proteins (hyaluronate synthetases). They are metalloproteins consisting of metal cations and glucoside phosphates. Hyaluronate synthetases are the only enzymes that catalyze acid production.
The process of destruction of C₂₈H₄₄N₂O₂₃ molecules occurs under the action of hyaluronate-lytic enzymes. There are at least seven of them in the human body, and some of them suppress tumor formation processes. The breakdown products of hyaluronic acid are oligo- and polysaccharides, which stimulate the formation of new blood vessels.
Introduction
Osteoarthritis (OA) is a joint disease, the main pathogenesis of which is cellular stress and degradation of the extracellular matrix. They arise as a result of macro- or microdamage that activates pathological adaptive recovery reactions, including pro-inflammatory pathways of the immune system [1]. According to estimates by the Federal State Statistics Service of the Russian Federation for 2022, diseases of the musculoskeletal system occupy a significant place in the structure of disability along with cardiovascular diseases and cancer. OA plays an important role among these diseases [2]. Currently, the prevalence of the disease is steadily increasing. OA accounts for 36 million outpatient visits and 750 thousand hospitalizations per year. In developed countries, the economic costs of OA amount to 1.5–2% of GDP per year [3]. Clinical manifestations and progression of OA do not pose a threat to the life of patients, but pain, which is a constant symptom of the disease, and limitation of joint function lead to a marked deterioration in the quality of life, the development of temporary and permanent disability. Against this background, there is a progression of concomitant diseases, which leads to an increased risk of overall mortality. In 2016, the Osteoarthritis Research Society International (OARSI), together with the US Food and Drug Administration (FDA), recognized OA as a serious disease [4]. In Russia, the prevalence of OA is also high and reaches 13%. According to the Russian Ministry of Health, over 5 years (2011–2016) there was an increase in the incidence of OA from 32.3 to 35.7 per 1000 population. In recent years, in the process of studying OA, ideas about its pathogenesis have changed significantly. Currently, OA is considered not as a degenerative disease, but as an inflammatory disease. The diversity and varying degrees of severity of individual pathogenesis links have led to the identification of several OA phenotypes. Changing understanding of the pathogenesis of the disease is also changing approaches to therapy, with an emphasis on personalized treatment.
Functions in the human body
Collagen and hyaluronic acid in human skin are the most valuable substances on which the elasticity and smoothness of the dermis depends. C₂₈H₄₄N₂O₂₃ performs the following functions:
- water conservation, which ensures the elasticity of the skin and its turgor;
- creating the required degree of viscosity of the intercellular fluid;
- participation in the reproduction of basic and immunocompetent cells of the epidermis;
- maintaining growth and restoration of damaged skin areas;
- strengthening collagen fibers;
- strengthening local immunity;
- protection against the influence of free radicals, chemical and biological agents.
The highest concentration of this substance is observed in the skin of the embryo. With aging, most of the acid is bound by proteins, which causes the level of hydration of the skin to decrease. The ability to self-regulate metabolism decreases especially strongly in people over 50 years of age.
The following properties of hyaluronic acid in synovial fluid have also been determined:
- formation of a homogeneous structure to retain a specific component of cartilage tissue - chondroitin sulfate;
- strengthening the collagen framework of cartilage;
- ensuring lubrication of the moving parts of the joints, reducing their wear.
The biological role of acid molecules differs depending on their molecular weight. Thus, compounds containing up to 1500 monomers have an anti-inflammatory effect and take an active part in building the collagen network. Polymers with a chain of up to 2000 monomers play a role in maintaining hydrobalance, and high molecular weight compounds have the most striking antioxidant properties.
Hyaluronic acid is also involved in the formation and development of the embryo, in the control of cell motility - the migration of cells from one place to another, in some interactions with surface cell receptors.
Hyaluronic acid: structure, synthesis, role in maintaining the structure and functions of joints
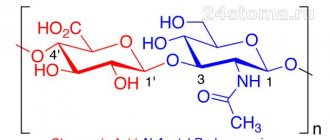
For the first time in 1934, Karl Mayer and John Palmer described the glycosaminoglycan they isolated from the vitreous body of the eye, proposing the name “hyaluronic acid”. Currently, this macromolecule is most often called "hyaluronate, hyaluronan", which reflects the fact that in vivo it exists as a polyanion and not in the form of a protonated acid. Hyaluronate is composed of disaccharide repeats of D-glucuronic acid and D-Nacetylglucosamine, which are linked through alternating beta-1,4 and beta1,3 glycosidic bonds. This structure is energetically very stable. The number of disaccharide repeats forming one hyaluronate molecule can be enormous (more than 30,000), and the molecular weight can exceed 107 Da. The average length of one disaccharide is about 1 nm; for example, a hyaluronate molecule of 10,000 repeats has a length of about 10 μm, which is comparable to the diameter of a red blood cell. The hyaluronate molecule is presented in different planes, has both hydrophobic and hydrophilic regions, and in physiological solution it folds in the form of a twisted ribbon with smooth bends in two planes, forming a rather large domain.
Schematic diagram of the structure of the hyaluronate molecule
Rice. 1 – Schematic structure of the structure of the hyaluronate molecule. The structural “building blocks” of which hyaluronate consists are disaccharide repeats of D-glucuronic acid and DN-acetylglucosamine, connected through alternating beta-1,4 and beta-1,3 glycosidic bonds (B), energy the stability of the molecule is very high. As noted above, in solution the hyaluronate molecule folds into a twisted ribbon, forming domain (B); water, electrolytes, amino acids and sugars freely penetrate into the domain, while at the same time protein molecules can enter the domain with great difficulty. Almost every human tissue contains hyaluronan. In cartilage, synovium and synovial fluid there is a relatively small amount of hyaluronate in absolute value (1 mg/g in hyaline cartilage, 3-4 mg/ml in synovial fluid), but it is an essential component of the matrix. Hyaluronate is found in the body of all vertebrates. Despite the fact that the structure of hyaluronate is quite simple, this glycosaminoglycan has several very important functions: – affects the interaction of cells with the intercellular substance and affects cell migration. Hyaluronate is one of the key components in processes associated with cell migration, such as embryogenesis, wound healing, inflammation and metastasis. Many of these processes are under the control of hyaluronate receptors, the most studied of which is the glycoprotein CD44 (a transmembrane receptor associated with the cytoskeleton) [20]. Hyaluronate may also mediate the association of aggrecan with the surface of chondrocyte cells via the CD44 receptor - hyaluronate plays a key role in retaining aggrecan within the cartilage matrix. Many aggrecan molecules bind non-covalently to a single hyaluronate molecule, forming very large complexes. Aggrecan is a high molecular weight proteoglycan specific for cartilage tissue. The binding of aggrecan to hyaluronan occurs in the extracellular space and does not require the participation of enzymatic systems. As part of synovial fluid, hyaluronate can serve as a lubricant (a substance that provides surface lubrication). In arthritis, the mass and length of hyaluronate molecules decrease.
Metabolism of hyaluronic acid in the body
Hyaluronan in the body is constantly renewed. Maintaining the optimal state of the cartilage matrix depends on the coordinated regulation of the synthesis and breakdown of this glycosaminoglycan. Hyaluronate is synthesized in the following way. Hyaluronate synthase enzymes (HAS proteins) are located in the plasma membrane of cells, and the resulting hyaluronate polymer directly enters the pericellular space, bypassing the endoplasmic reticulum and the Golgi complex. Thus, unlike other glycosaminoglycans synthesized in the Golgi apparatus, hyaluronic acid is synthesized on the inner surface of the plasma membrane. As the polymer chain lengthens, HA is released through the membrane to its outer surface. Outside the cell, HA can form complexes with hyaluronate-binding proteins called hyalatherins. Hyaluronate synthesis is ensured by HAS proteins. Three such proteins have been identified, of which HAS2 is the most abundant: • The HAS1 and HAS2 proteins mediate the synthesis of high molecular weight hyaluronate. When the HAS2 function is blocked in human chondrocytes, the assembly of the extracellular matrix is disrupted and proteoglycans are lost from the pericellular space, while the viability of the cells themselves is not impaired • HAS3 synthesizes molecules of shorter length. Hyaluronate molecules of different lengths have different effects on cell behavior. Very short chain length hyaluronans stimulate cell proliferation and may be involved in angiogenesis and inflammation. High molecular weight hyaluronans, on the contrary, inhibit proliferation. Studying the mechanisms of regulation of hyaluronate synthase has outlined new approaches to the treatment of conditions that require high levels of hyaluronic acid. It has been shown that compounds that activate protein kinase C (for example, phorbol esters) can increase the synthesis of hyaluronate in mammalian cells. Compounds that stimulate protein kinase A also increase hyaluronate biosynthesis. Hyaluronic acid is widely distributed in tissues, present in high concentrations in synovial fluid, the vitreous humor of the eye, and in the connective tissues of chicken combs, umbilical cord, and dermis (Table 1).
Table 1. Tissues with the highest concentration of Hyaluronic acid
| Body tissue | Concentration of hyaluronic acid (according to Hascall VC and Laurent TC, 1997, with additions) |
| amniotic fluid (16-20 weeks of pregnancy) | 20 mg/ml |
| chicken combs | up to 7.5 mg/ml |
| umbilical cord | 4 mg/ml |
| synovial fluid | 3-4 mg/ml |
| hyaline cartilage | 1 mg/g wet tissue |
| dermis | 0.5 mg/g wet tissue |
| vitreous body of the human eye | 0.1-0.4 mg/g wet tissue |
| epidermis | 0.1 mg/g wet tissue |
Biological role of hyaluronic acid
Together with other proteoglycans, HA is part of the intercellular matrix of the skin. Due to its physicochemical properties (high viscosity, specific ability to bind water and proteins and form proteoglycan aggregates), HA contributes to the manifestation of numerous functions of connective tissue (Table 2). Table 2. Functions of connective tissue.
| Biomechanical | Physical activity. HA is part of the cartilage that covers the rubbing surfaces of joints. |
| Trophic | Active metabolism between blood and tissues. By forming intercellular spaces, HA facilitates the supply of nutrients to cells and the removal of metabolic products. |
| Barrier | Protection from external influences. GC modulates the functional state of phagocytes and immunocompetent cells. |
| Plastic | Regeneration and replacement of defects. By interacting with cell surface receptors, HA stimulates fibroblast migration and cell proliferation. |
| Morphogenetic | Formation of the structure of organs and tissues in embryogenesis and the postnatal period. |
Biomechanical Motor activity. HA is part of the cartilage that covers the rubbing surfaces of joints. Trophic Active metabolism between blood and tissues. By forming intercellular spaces, HA facilitates the supply of nutrients to cells and the removal of metabolic products. Barrier Protection from external influences. GC modulates the functional state of phagocytes and immunocompetent cells. Plastic Regeneration and replacement of defects. By interacting with cell surface receptors, HA stimulates fibroblast migration and cell proliferation. Morphogenetic Formation of the structure of organs and tissues in embryogenesis and the postnatal period. Hyaluron helps the skin maintain its elasticity and youth by stimulating collagen production. Hyaluronic acid is important in maintaining the normal state of the structure of the joints and maintaining their functions. Joints are movable joints of skeletal bones, separated by a gap, covered with a synovial membrane and an articular capsule. The joints are located in the skeleton in places where distinct movements occur: flexion (flexio) and extension (extensio), abduction (abductio) and adduction (adductio), pronation (pronatio) and supination (supinatio), rotation (circumductio). As an integral organ, the joint takes an important part in supporting and motor functions. Each joint is formed by the articular surfaces of the epiphyses of the bones, covered with hyline cartilage, the articular cavity containing a small amount of synovial fluid, the joint capsule and the synovial membrane. In the cavity of the knee joint there are menisci (cartilaginous formations that increase the congruence (compliance) of the articular surfaces, which are additional shock absorbers that soften the effect of shocks). There are several classifications of joints; the most obvious one to describe the structure is the knee joint.
Fig. 2 – Diagram of the structure of the knee joint
Diagram of the structure of the knee joint
Articular cartilage is specialized connective tissue that covers the contacting surfaces of synovial joints. The main functions of cartilage are to allow movement between bones with minimal friction and maximum speed, absorb forces transmitted during movement, and maintain joint stability. Synovial fluid plays a key role in ensuring lubrication of the moving surfaces of joints. Synovial fluid. The volume and composition of synovial fluid is determined by the properties of the synovium and its blood supply. In normal joints, fluid is contained in small quantities (2.5 ml in the knee joint), sufficient to cover the synovial surfaces with a thin film, but not to separate the surfaces. Synovial fluid provides not only lubrication, but also nutrition to the avascular structures of the joint, including joint cartilage and tendon sheaths. The protein content in synovial fluid is inversely proportional to their molecular weight; albumin content is about 45% of its concentration in blood plasma. Fibrinogen, macroglobulins and complement components practically do not enter the synovial fluid. The total protein concentration in normal synovial fluid is 1.3 g/dL. The concentration of electrolytes and small molecules in synovial fluid is equivalent to that in blood plasma. With osteoarthritis, there is a violation of the homogeneity of the articular surfaces, separation and fissures of hyaline cartilage, and even its erosion with exposure of the underlying bone. Marginal osteophytes are formed, representing bone overgrowth, covered with newly formed irregular hyaline and fibrous cartilage. Hyaluronate (hyaluronic acid) is synthesized by fibroblast-like cells of the synovial membrane and in synovial fluid it is found in a concentration of about 3 g/l, which is many times higher than the content in blood plasma - about 30 μg/l. The volume of synovial fluid mainly depends on the amount of hyaluronate; one of the main functions of this glycosaminoglycan is considered to be water retention. Hyaluronate can trap various molecules in the synovial cavity, acting as a filter on the surface of the synovium, limiting the exit of fluid from the joint cavity. The synovial fluid itself and its protein components have a short turnover time (about 1 hour in a normal knee joint). However, the rate of hyaluronate turnover under normal conditions is much lower (about 13 hours), due to which it can serve as a “trap” for many molecules. Another component of synovial fluid that provides lubrication of articular surfaces, the glycoprotein lubricin, is also synthesized by the cells of the synovial membrane. Unlike hyaluronate, lubricin is thought to provide marginal lubrication. During inflammation, an increase in protein concentration in the synovial fluid is observed. Increased vascular permeability during inflammation causes improved permeability for all proteins, but especially noticeable changes affect high molecular weight proteins. At the same time, permeability to water, electrolytes and small molecules does not change during inflammation. Thus, synovial inflammation leads to a marked increase in protein concentrations without adequately increasing the supply of nutrients and removing waste products. The joint cavity is normally a collapsed cavity containing a small amount of fluid distributed over the articular surfaces. This collapsed state is maintained by the subatmospheric pressure of the joint cavity. Under normal conditions, intra-articular pressure is maintained at rest at a level slightly below atmospheric (0 to -5 mmHg). During movements, a further decrease in hydrostatic pressure may be observed. The ease with which movement occurs at a joint depends on the force applied, the characteristics of the opposing surfaces, and the properties of the material between the surfaces. It is hyaluric acid in the synovial fluid that ensures the creation of optimal conditions for reducing the coefficient of friction and provides thin-film lubrication of the articular surfaces. The exact mechanism for maintaining subatmospheric pressure in the joints is currently not fully understood. It is hypothesized that the high colloidal osmotic pressure in the pericapsular gel-like layer may provide negative hydrostatic pressure within the capsule. To maintain this balance, it is necessary to fix glycosaminoglycans, primarily hyaluronate, in the gel layer covering the articular surfaces. The introduction of hyaluronidase leads to the destruction of the gel and an increase in intra-articular pressure. Hyaluronate is the basis of a molecular “sponge” that adsorbs water and provides negative hydrostatic pressure. In chronic arthritis, there is an increase in intra-articular pressure due to an increase in the volume of synovial fluid and a decrease in the extensibility of the joint capsule. In the presence of joint effusion, repeated mechanical stress can lead to impaired synovial perfusion. The lowest pressure in the presence of effusion is observed when the joint is flexed at about 30 degrees. Full flexion and extension of the joint increases pressure and causes significant subjective discomfort, which, in turn, leads to limited range of motion. Increased pressure can cause pathological protrusions of the joint capsule, and even a violation of its integrity. Thus, hyaluronic acid plays a very important role for a person and his joints, being responsible for the viscosity of the synovial fluid, ensuring sufficient sliding of the cartilage for the mobility, flexibility and elasticity of the joint itself. This substance is involved in the nutrition of joint elements and helps maintain joint mobility even with a high percentage of destruction. Retaining water in cartilage is also one of the main tasks of hyaluronic acid, which increases the elasticity and flexibility of the joint. With age, the production of hyaluronate in the body noticeably decreases, this can be seen in the condition of the skin and joints.
Hyaluronic acid in the treatment of joint diseases
People with joint problems due to illness or age may need treatment. Thus, osteoarthritis is an important medical and social problem. The incidence of osteoarthritis in Russia is 13% among the adult population. Moreover, in 68% of cases, the knee and hip joints are most often affected. Therapy for osteoarthritis includes non-drug and drug methods, taking into account the individual characteristics of the patient. In recent years, hyaluronic acid preparations have found widespread use for osteoarthritis of the knee joints. Hyaluronic acid is an effective treatment for joint diseases, since it is a natural main component of intra-articular fluid, a kind of joint lubricant that protects it from injury. It has been confirmed that intra-articular injections of hyaluronic acid preparations are more effective than placebo, are not inferior to traditional methods of treatment and can be used as symptomatic therapy for gonarthrosis. Treatment with hyaluronic acid preparations leads to a reduction in pain and improvement in function; it is indicated for patients with osteoarthritis of the knee joints of radiological stages, under the age of 65 years, with pain that is not relieved by therapy with non-drug drugs and simple analgesics, and who have contraindications to the use of non-steroidal drugs. anti-inflammatory drugs. Joint injections with hyaluronic acid make the intra-articular fluid more dense and viscous, providing better protection for cartilage tissue. In addition, it relieves inflammation and accelerates the process of cartilage restoration. Such injections are often prescribed for arthrosis of the joints. Hyaluronic acid preparations of different molecular weights have comparable effectiveness. Tolerability of therapy is satisfactory; repeated courses do not increase the risk of side effects. Ointments with hyaluronic acid are characterized by lower bioavailability and are more often used as an auxiliary therapy. Oral preparations with hyaluronic acid are dietary supplements aimed at preventing the disease and are recommended as an additional source of hyaluron for the body.
Receipt
There are 2 main groups of methods for obtaining the substance:
- Physico-chemical (extraction from tissues of mammals, vertebrates and birds). Since acid in animal raw materials is often contained in a complex with proteins and other polysaccharides, careful purification of the resulting product is required, which affects the cost of the final product. To obtain acid on an industrial scale, the umbilical cord of newborn children and the combs of domestic chickens are used. There are other methods of extraction - from the eyes of cattle, the liquid that fills the cavities of the joints and joint capsules; blood plasma, cartilage, pig skin.
- Microbial methods based on bacteria cultivated in a nutrient medium. The main producers are Pasteurellamultocida and Streptococcus bacteria. These methods were first tested in 1953. They are more economical and do not depend on seasonal supplies of raw materials.
In the first case, biological materials are destroyed by grinding and homogenization methods, and then the acid mixed with peptides is extracted through exposure to organic solvents. The resulting mass is treated with enzymes or proteins are removed by denaturation using chloroform or a mixture of ethanol and amyl alcohol. After this, the substance is concentrated on activated carbon. Final purification is done by ion exchange chromatography or precipitation with cetylpyridinium chloride.
Anti-inflammatory and regenerating mechanisms of action of GCs
The physiological role of HA has been well described by many researchers [8–10]. In general, HA can participate in various cellular processes (cell differentiation, proliferation, etc.) and perform physiological functions (lubrication, hydration balance) [9, 10]. HA has unique rheological properties and is an integral part of articular cartilage; the important role of HA in the functioning of joints, not only healthy ones, but also joints with OA [11], as well as other body tissues, has been well studied [12]. Studying the pharmacological action of GC, E. Maheu [13], GM Campo [14] and J. Jerosch. [15] in their studies proved that GC reduces the activity of proinflammatory mediators that produce neuropeptides released by activated synovial cells. The authors also described the interaction of HA with pain receptors and its analgesic properties.
LW Moreland [10] noted in his study that HA can reduce the mechanosensitivity of pain receptors. GC may also reduce OA pain by reducing the synthesis of prostaglandin E2 and bradykinin.
In OA, synovitis plays an important role in inducing pain, swelling and stiffness [16], and the severity of synovitis correlates with a high risk of progression to knee OA [17]. A study was conducted that showed that GC inhibits the activity of matrix metalloproteinases and aggrecanases, which are at least partially involved in the degradation of OA cartilage as a result of their induction by proinflammatory cytokines such as interleukin-1 (IL-1) [18]. . Therefore, it is hypothesized that GC modifies structural joint damage and the rate of progression of OA in addition to its symptom-modifying effect [19].
Regarding the reparative function of HA, JL Cook et al. [20] suggested that intra-articular administration of GC has a direct effect on chondrocytes or synoviocytes and the production of Transforming growth factor beta (TGF-β), basic fibroblast growth factors (FGF) and insulin-like growth factor (Insulin-β). like growth factor, IGF). Histological data confirmed that HA prevents cartilage degradation and may promote cartilage regeneration. Other authors [14] have also provided evidence that GC treatment mitigates synovial hypertrophy and increases the number of synovial fibroblast-like cells while reducing the number of macrophages, lymphocytes, mast cells and adipocytes. HA, according to them, provides cartilage protection by suppressing the production of proinflammatory cytokines, free radicals and proteolytic enzymes in the synovial fluid.
A large number of clinical studies have found that GC reduces the progression of OA. GC is a slow-acting drug that can be used prophylactically or therapeutically as an anti-inflammatory symptom-modifying drug [20, 21].
Several clinical studies have found that intra-articular HA injections slow joint space narrowing on x-ray and stop the progression of cartilage degeneration on subsequent arthroscopy [22, 23].
Use in medicine
Hyaluronic acid is used for the following pathologies:
- ophthalmology – cataract; use as a surgical environment during operations;
- orthopedics – osteoarthritis, protection of articular cartilage from destruction, as well as to stimulate its recovery (synovial fluid endoprostheses);
- surgery - increasing the volume of soft tissue, operations with extensive excision of cartilage tissue;
- pharmaceuticals – production of drugs based on the polymer structure of the compound (tablets, capsules, creams, gels, ointments);
- food industry – sports nutrition;
- gynecology – anti-adhesion agents;
- dermatology – treatment of burns, post-thrombotic trophic skin disorders.
According to scientists' forecasts, this substance could become the basis for a new group of drugs for the treatment of cancer.
Other properties of the acid are also promising:
- antimicrobial, antiviral effect (the compound is active against the herpes virus and others);
- improvement of blood microcirculation;
- anti-inflammatory effect;
- prolonged action (gradual dissolution in human tissues).
Properties of preparations based on hyaluronic acid
All hyaluronic acid options are biodegradable. Since this substance is found in many tissues of our body, for its utilization nature provides the enzyme hyaluronidase, which completely decomposes and removes hyaluronic acid molecules.
During its stay in the tissues, hyaluronic acid manages to moisturize and nourish them. By influencing fibroblast cells that produce skin proteins, it triggers rejuvenating processes. Rejuvenation occurs due to the internal reserves of the body, therefore it does not produce consequences, and the resulting effect lasts for a long time.
Understanding the various characteristics and capabilities of products with hyaluronic acid allows you to navigate their diversity. It will be much easier to choose the best option to achieve the desired result.
Vitamins
Hyaluronic acid in vitamins is used in the form of purified sodium hyaluronate, which is its analogue. The main purpose of the substance is to preserve the youth of the skin, moisturize it, and heal wounds. To improve absorption, ascorbic acid is added to vitamin complexes.
Research is also underway to develop drugs and dietary supplements with anti-inflammatory and immunomodulatory effects, which can be used in many areas of human activity.
Recommendations for the management of patients with OA
Currently, OA therapy is regulated by documents developed by professional communities. ESCEO (European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases) has developed guidelines that guide consistent treatment choices for patients with knee OA . They were presented in 2014 [5], and supplemented in 2016 [6]. New recommendations were published in 2022, which take into account the latest evidence on the safety and effectiveness of drugs currently used to treat OA [7]. The developed scheme recommends step-by-step administration of therapy (Fig. 1).
If OA symptoms persist, the use of hyaluronic acid (HA) is indicated, both as monotherapy and in combination with drugs from other groups. Let's take a closer look at the effects of HA.
Cosmetology
In cosmetology, this compound is used to correct age-related changes. Because the acid structure is similar in all living organisms, it is suitable for use as a dermal filler (injectable), especially around the eyes. In order for the substance to remain longer in the epidermis, it is modified using cross-linking molecules (crosslinkers). “Cross-linked” fillers differ from each other in gel viscosity, acid concentration, and duration of resorption in the skin.
Injections are administered intra- or subcutaneously in the form of a 1-3% aqueous solution. This helps to increase the elasticity and firmness of tissues and noticeably smooth out wrinkles.
C₂₈H₄₄N₂O₂₃ is also added to external cosmetics - gels, foams, creams and other basic products. Hyaluronic acid in the composition is designated as hyaluronic acid (and sodium hyaluronate is sodium hyaluronate). This type of cosmetic product has the same properties as fillers - it prevents the formation of wrinkles and acne, and helps saturate the skin with moisture.
Application in cosmetology
Hyaluronic acid has gained the greatest popularity in aesthetic medicine. The main indication for prescribing HA injections in cosmetology is the need to correct various deformations of skin contours, such as:
- scarring,
- deep wrinkles,
- folds,
- changing the shape of the lips.
Giving the lips additional volume by introducing hyaluronic acid does not have a lifelong effect.
The drug is administered intradermally, and the visible volume of the tissue increases. The maximum dose for correction of one area should not exceed 30 mg of active substance or 1.5 ml. If the patient requires a larger volume of the drug, then the injections are given in separate courses.
Side effects of this procedure may include:
- swelling,
- redness,
- itching,
- pain at the injection site,
- acne-like papules,
which usually go away on their own within a day or two. It should be taken into account that the administration of GC is incompatible with the use of other drugs, and when taking anticoagulants it can lead to the appearance of hematomas. There are also contraindications for the use of HA. So, hyaluronic acid cannot be injected:
- with skin hypersensitivity;
- after laser and chemical peeling;
- if there is inflammation in the area where injection is necessary;
- pregnancy and breastfeeding;
- severe chronic diseases;
- autoimmune diseases;
- tendency to form keloid scars;
- acute form of herpes.
GC preparations should not be used before the age of 25
Biorevitalization of the face
A procedure called biorevitalization is performed in beauty salons for the purpose of rejuvenation. To correct wrinkles and folds, HA is injected into the skin linearly or a series of pinpoint injections are performed. The effect of this procedure is to moisturize the skin and rejuvenate its appearance due to the stimulation of the production of its own collagen and elastin fibers.
The technique can be used for any area of the skin that requires correction, but most often it is required in open areas exposed to ultraviolet radiation. This is the face, neck, décolleté and hands. A course of biorevitalization can be preventive or therapeutic.
The first is used to prevent dryness and early aging of the skin and includes two procedures with an interval of 3-4 weeks. This course moisturizes the skin well and helps normalize metabolic processes, thus preventing the withering of exposed skin. Typically used on the face, lips and hands.
Indications for therapeutic revitalization include the presence of pronounced age-related skin changes. The treatment course involves a deeper and more intense effect on the skin and includes three procedures performed at a similar interval. The treatment procedure effectively copes with such changes as:
- severe dehydration and aging of the skin;
- violation of skin pigmentation;
- decreased elasticity and skin turgor;
- recovery after plastic surgery and aggressive peeling.
For two days after the procedure, you should not touch your face, much less use cosmetics. The exception is those medications prescribed by the doctor. For a week after the procedure, you should not go to the bathhouse or sauna or play sports. Within two weeks from the date of injection, the skin should be protected from exposure to ultraviolet radiation.
Reviews from those who have undergone this procedure confirm its effectiveness and quick results, although many find it quite painful. In this case, bruises and papules can persist for up to three days.
In addition to the solution for intradermal injection, produced in ampoules, hyaluronic acid is included in various anti-aging creams, gels, and masks, which are used both in beauty salons and at home.
Application methods for using HA are available at home