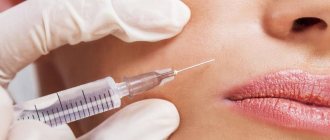
The Radiesse™ Injectable Implant is a steam sterilized, latex-free, pyrogen-free, semi-solid, cohesive, fully biodegradable deep dermal and subdermal implant. The main component is synthetic calcium hydroxylapatite, a biomaterial that has been used in orthopedic practice, neurosurgery, dentistry, otolaryngology and ophthalmology for more than twenty years.
Calcium hydroxylapatite is the main mineral constituent of bones and teeth. The semi-solid structure of the implant is formed by creating a suspension of calcium hydroxylapatite based on a gel consisting primarily of water (sterile water for injection (USP)) and glycerol (USP). The gel structure is obtained by adding a small amount of sodium carboxymethylcellulose (USP). The gel is absorbed in vivo and replaced by growing soft tissue, while calcium hydroxylapatite remains at the injection site. As a result, long-term, but not permanent, restoration and growth is achieved.
The Radiesse™ injectable implant is classified as a medical device. Radiesse™ Injectable Implant 1.5 cc, 0.8 cc, and 0.3 cc have particle sizes of 25-45 microns and can be injected with a 27-gauge or larger needle with a standard luer lock. Use of needles smaller than 27 gauge may increase the risk of needle blockage
.
Contraindications
- A contraindication is the presence of acute and/or chronic inflammation or infection at the site of the procedure.
- The drug is contraindicated in patients with hypersensitivity to any of the components.
- Contraindicated for patients prone to inflammatory skin reactions or patients with a tendency to hypertrophic scars.
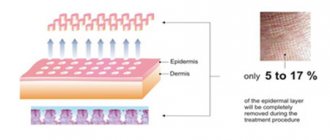
- Do not implant the product into the epidermis or use as a skin substitute. Implanting it into the epidermis or superficial dermis can lead to complications such as fistula formation, infections, extrusions, nodule formation and hardening.
- Not intended for use for the correction of glabellar folds. A higher risk of local necrosis is associated with glabellar infection. Complications associated with other injectable agents indicate that intensive injections into the superficial dermal vessels of the glabellar region may cause retrograde movement into the retinal arteries, which may lead to vascular occlusion.
- Contraindicated in the presence of foreign bodies, for example, liquid silicone or other fractional materials.
- Should not be used in areas with insufficient coverage of healthy, well-vascularized tissue.
- Should not be used in patients with systemic disorders that may result in poor wound healing or tissue damage over the implant.
Indications for use of the drug Radiesse
Radiesse can be used for the following indications:
correction of the nasolabial fold between the nose and mouth
correction of creases in the corners of the lips
correction of depressions and marks from scars, acne
contour plastic surgery (increasing the volume or changing the shape) of the cheekbones, cheeks, caused by a lack of soft tissues of the face or bone structures
correction of the shape of the nose, if necessary, with the creation of additional volume
correction of the shape and increase in the size of the chin
improvement of the contours of the lower jaw
The drug is used not only on the face, but also on the body, for example, to reduce the appearance of veins and bones, noticeable with age on the hands, to reinforce weakened tissues of the inner surface of the arms, thighs, and above the knees. The drug has proven itself well in combination with rejuvenating procedures using the Elos device. The use of combined techniques allows you to increase the level of collagen production and achieve high and prolonged results of anti-aging therapy.
Mesoradis, what is it?
There is a mesoradiesse technique, when the Radiesse drug is diluted 3-5 times with 0.9% NaCl and injected into the dermis in liquid form. This technique is used when the skin appears atonic, sagging, or decreased in its density. Such signs of aging can appear after 40 years in the face, neck, décolleté and hands.
The MesoRadiesse technique is the reinforcement of the skin with calcium hydroxyapatite. The drug is diluted with saline to the required consistency to penetrate the more superficial layers of the skin, and is laid with thin “threads”.
The procedure has a lasting rejuvenating effect by enhancing the skin's production of its own collagen and elastin, increasing skin tone and elasticity, and reducing facial wrinkles and folds.
Meso-Radiesse is the key to youth.
This unique technique serves to restore the synthesis of your own collagen and elastin. As a rule, the course is from 1 to 3 procedures with an interval of 3-4 months. The Radiesse drug is administered along vectors - Langer lines (tension lines corresponding to the direction in which the collagen fibers are located).
The drug is also recommended to prevent a decrease in skin turgor and tone after 35 years.
One procedure combines different techniques for administering drugs: bolus to the depth of the periosteum in the cheekbone area, vector in the middle and lower third of the face and mesoradiess to the entire surface of the skin. Thus, in one procedure a comprehensive lifting effect is achieved, as a result of which the face looks fresher, more rested, and younger. Sometimes the drug is used to increase bone mass during lipofilling.
Radiesse is an absorbable filler, like hyaluronic acid-based products, but more long-lasting in its results. Due to the intense stimulation of collagen, even after the filler is completely removed, synthesized collagen fibers remain in the skin, which firmly hold the “framework” of the skin and prevent the facial tissue from moving downwards.
Radiesse is the drug of choice for men who need an invisible, most natural correction of age-related facial changes. Radiesse allows you to accentuate the cheek area, highlight the mandibular angle and chin, which will create a more masculine appearance and will not make the face round (which is what hyaluronic acid does).
Long-term effectiveness (more than 12 months) and safety of use, proven by numerous studies, are an additional reason for choosing this particular drug.
Warnings
- The implant should not be inserted into blood vessels. Injection into blood vessels may cause platelet aggregation, vascular occlusion, infarction, embolism, or hemolysis.
- Should not be injected into organs or other structures that may be damaged by the space created by the implant.
- Implantation should not be performed on patients taking aspirin or other medications that may interfere with the healing process.
- Should not be implanted into infected or potentially infected tissue or into open cavities as infection or extrusion may occur. Significant infection can cause damage or loss of skin over the implant. Hematomas or seromas may require surgical drainage.
- In the event of a hypersensitivity or allergic reaction, significant inflammation or infection may occur requiring removal of the implant.
- Some injectable implants have caused hardening of tissue at the injection site, migration of particles from the injection site to other parts of the body, and/or allergic or autoimmune reactions. Based on clinical use, animal studies and relevant literature, these reactions have not been observed and are not expected to occur with the Radiesse™ Injectable Implant.
- As with any implant material, possible side effects may include, but are not limited to: inflammation, infection, fistula formation, extrusion, hematoma, seroma, calcification, problematic healing, skin discoloration, and under- or over-augmentation.
- The safety and effectiveness of the product for women during pregnancy or lactation have not been established.
- The safety and effectiveness of the Radiesse™ injectable implant for use in the labial mucosa has not been established.
How does Radiesse™ work?
Radiesse™ consists of microspheres of synthetic calcium hydroxyapatite (CaHA) (30%) suspended in an aqueous gel carrier (70%).
Calcium hydroxyapatite (CaHA) particles are produced from sub-micron grains of the parent material. These grains are combined during high temperature processing into microspheres
- CaHA (calcium hydroxyapatite) microspheres have the same shape, the size varies from 25 to 45 microns in diameter
- Ca2+ and PO3- ions are natural components of teeth and bones, making them known to be safe and biocompatible
- Calcium hydroxyapatite CaHA in the Radiesse™ filler is not bone, it is an inorganic component of the tissue of teeth and bones
- The carrier gel holds the microspheres together.
Precautionary measures
- Soft tissue is required for easy percutaneous insertion of the Radiesse™ injectable implant. Difficulties may arise when inserting an implant into scar tissue and tissue with significant damage.
- An infection may occur at the injection site that requires treatment. If such an infection cannot be treated, the implant may need to be removed.
- Injection-related reactions, including seroma, swelling, pain, itching, discoloration, or tenderness, may also occur at the injection site. They usually disappear on their own within one to two days after the injection.
- Nodules may form that require treatment or removal.
- There may also be uneven distribution of the implant, requiring surgical correction.
- Do not inject excessive amounts of implant. In extreme cases, the skin at the injection site may burst. The Radiesse™ injectable implant can be added to later injections, but is difficult to remove.
- The Radiesse™ Injectable Implant procedure, like similar injection procedures, has small but inherent risks of infection and/or bleeding. The patient may experience mild discomfort during and after the procedure. Therefore, it is recommended to use anesthetic techniques for this therapeutic procedure. Standard precautions associated with subcutaneous injections should be followed to prevent infection.
- Cannot be re-sterilized
. The Radiesse™ Injectable Implant is supplied sterile and pyrogen-free in a sealed foil pouch for single-patient use only. - The foil package should be carefully inspected to ensure that the packaging and syringe have not been damaged during transportation. Do not use the product if the package is opened or the syringe is damaged. Do not use if syringe cap or plunger is removed or dislodged. For sterilization, there is usually a small amount of moisture in the foil bag; this is not a sign of damage.
Composition of the drug
Radiesse is an injectable preparation that provides long-term correction of scars from 18 months. The unique Radiesse filler contains 30% calcium hydroxyapatite microspheres and 70% carrier gel, so the drug is 100% biocompatible.
Immediately after injection, the missing volume is filled and wrinkles are smoothed out. After 2-3 weeks, calcium microspheres stimulate the production of new collagen, which improves the quality of the skin and the effect lasts for a long time.
Radiesse has been approved by the FDA as generally safe (GRAS) since 1995 in the United States for:
- correction of facial lipoatrophy (tissue volume deficiency, ptosis);
- correction of protruding facial folds, including nasolabial folds, loss of skin tone.
Later in 2003, Radiesse was approved in the European Union for use in plastic and reconstructive surgery, including deep and superficial grafting of soft tissue defects of the face and other parts of the body.
Individualization of therapy
Before the procedure, the patient's suitability for therapy should be assessed, as well as the patient's need for anesthesia. The outcome of therapy depends on the patient. In some cases, additional therapy may be required, depending on the size of the defect and the patient's needs. Additional injections may be given, but only after sufficient time to evaluate the patient's condition. Repeated injections should not be performed earlier than seven days after previous therapy.
Photos before and after
Correction of facial oval Radiesse, before and after photos (doctor N.A. Solovykh)
Correction of facial oval Radiesse, before and after photos (doctor N.A. Solovykh)
Correction of facial oval Radiesse, before and after photos (doctor N.A. Solovykh)
Correction of facial oval Radiesse, before and after photos (doctor N.A. Solovykh)
Vector lifting Radiesse (doctor Ratnikova S.V.)
Vector lifting Radiesse (doctor Ratnikova S.V.)
Vector lifting Radiesse (doctor Ratnikova S.V.)
PREPARATION FOR PRODUCT OPERATION
Directions for use
The following is required for the subcutaneous injection procedure:
- Radiesse™ Injectable Implant Syringe(s) (sold separately)
- Needle(s) of the required gauge with luer lock connectors (not included in delivery). The required needle size is 25 ga - 27 ga, 1/2 - 11/2 inches. The use of needles smaller than 27 gauge may increase the risk of needle blockage.
1) Prepare the patient for subcutaneous injection using standard techniques. The injection site should be marked with a surgical marker and prepared with an appropriate antiseptic. Local or topical anesthesia at the injection site or sedation should be used at the physician's discretion. After anesthesia, apply ice to reduce swelling/stretching.
2) Before subcutaneous injection, prepare syringes and needles. A new needle should be used for each syringe, or one needle may be attached to each new syringe for the same patient.
Remove the foil packaging from the box. If necessary, the package can be opened and the syringe placed in a sterile field. For sterilization, there is usually a small amount of moisture in the foil bag; this is not a sign of damage.
Correction of cheekbones with Radiesse filler
Beautiful high cheekbones give a youthful appearance. Today, it is only possible to “remove” such cheekbones without surgery using Radiesse.
Contour plastic surgery of the cheekbones with Radiesse gives a very good and long-lasting result, since the filler has a high viscosity, which is ideal for correcting this particular area. With just a few injections, the filler allows you to correct your cheekbones. This technique is characterized by the absence of a postoperative period and almost instant results.
With the help of Radiesse, new contours are formed not only of the cheekbones, but also of the cheeks, while taking into account the smallest anatomical features of the face.
It is noteworthy that Radiesse injections make it possible not only to enlarge, but also to make low cheekbones higher, and to turn thin and plump faces into normal ones.
PRODUCT OPERATION PROCEDURE
Remove the luer lock from the distal end of the syringe before attaching the needle. The syringe can then be screwed onto the luer lock of the needle. The needle must be securely attached to the syringe and filled with the injectable implant Radiesse™ (Radiesse)
. Excess implant on the surface of the Luer lock connectors should be wiped off with a sterile cloth. Slowly press the plunger of the syringe to release the implant material from the tip of the needle. If the luer lock is leaking, it may be necessary to remove the needle and clean the luer lock surfaces or, as a last resort, replace the syringe and needle.
3) Determine the implant insertion site. Injections may be difficult or impossible to perform in scar tissue and cartilage. When inserting the needle, be careful not to pierce these types of tissue if necessary.
DO NOT INJECT INTO A BLOOD VESSEL!
4) The depth of injection and the amount of material injected depend on the location and size of the restoration or augmentation. The Radiesse™ injectable implant should be inserted deep enough to prevent the formation of nodules on the skin surface or ischemia of the overlying tissue.
5) DO NOT OVER-CORRECTION THE INJECTION SITE. Apply a correction factor of 1:1. Smooth or massage the inserted implant periodically throughout the injection process to achieve a smooth contour.
6) If there is significant resistance when pressing the plunger, you can move the needle slightly to make it easier to insert the material. If you still feel significant resistance, you may need to remove the needle completely from the injection site and try again in a different location. If significant resistance continues to be felt, a different injection needle may need to be used. If the material cannot be injected, change the syringe and needle.
7) Insert the needle into the deep layer of the dermis until the desired position is achieved. (For information on augmenting specific areas of the face, see additional instructions below.) Gently depress the plunger of the syringe to begin inserting the implant and slowly inject the material, withdrawing the needle to place the strip of material in the desired location. Continue placing additional strips of material until the desired level of augmentation is achieved.
Augmentation of the cheeks, chin or corners of the mouth
- Insert the needle into the skin, bevel down, at an angle of approximately 30°. The needle should slide into the deep layer of the dermis to the point where you want to begin the injection. It should be easily palpable with your free hand.
- Apply slow, steady pressure to the plunger to insert the implant, while pulling the needle out to leave a thin, single strand of material. The implant material thread must be completely surrounded by soft tissue, without globular deposits.
- Individual strands of implant material should be placed parallel and close to each other, and also in layers if deep folds are being corrected. It is possible to cross-layer the threads in a deeper plane for structural support.
- After injection, use your index finger and thumb to smooth out these areas to better distribute the implant if any minor nodules of material form.
- It is possible to perform injections into the subcutaneous tissue or under the muscle, but not into the bone or epidermis.
PATIENT INFORMATION
The patient should be advised of proper post-procedure care, which may include the following recommendations to promote normal healing and avoid complications.
- Ice or cold compresses should be applied to the injection areas for approximately 24 hours.
- After the operation, it is necessary to avoid the sun, not to visit the solarium, sauna or intensive cosmetic procedures.
- It is necessary to massage the injection areas if nodules are detected during palpation.
- The patient should be advised of the common effects of swelling and numbness. The swelling usually disappears within 7-10 days, but can last up to several weeks. The numbness will go away in 4-6 weeks.
Provide the patient with oral analgesics and advise rinsing with saline 4 to 6 times daily for 1 week after surgery.
The advantages of this drug, and for which areas it is used
100% biocompatible. The drug contains no components of animal origin, which minimizes the possibility of allergic reactions. Does not add “extra volume”, the drug is not hydrophilic. Stimulates the production of your own collagen, creating the effect of velvety skin and unsurpassed lifting. It is thanks to neocalogenesis that the effect of the drug lasts 18 months or more.
The big advantage is that the drug is administered using a cannula. A cannula is a medical flexible tube with a rounded end through which a drug is administered. Unlike a needle, which, due to its sharp end, tears tissue and can injure blood vessels and nerve fibers in the insertion area, a cannula allows you to gently move apart tissue and bypass, for example, obstacles such as blood vessels, without injuring them. The rehabilitation period with previously known swelling and bruising after the cannula is practically absent, since the tissues retain their integrity.
Thanks to the use of Radiesse:
- First of all, the so-called V-effect is achieved, rejuvenating the surface;
- improves complexion;
- small wrinkles are smoothed out;
- sunken cheeks are eliminated;
- The oval of the face is tightened.
Correction can also be carried out on the décolleté and hands. So far, Radiesse is the only drug for high-quality correction of the zygomatic bone.
RULES FOR STORAGE AND USE
Supply
The Radiesse™ Injectable Implant is supplied sterile and pyrogen-free in a syringe packaged in a foil pouch and box for easy storage. Each kit consists of one filled syringe containing 1.5 cc, 0.8 cc, or 0.3 cc of Radiesse™ injectable implant. The degree of accuracy of calibration of syringe divisions is ±0.025 cm3. Do not use the product if the packaging and/or syringe is damaged or if the tip or plunger of the syringe is affected or dislodged.
The contents of the syringe are intended for one patient, for single use only and cannot be re-sterilized. Repeated use may impair the functionality of the device and cause the device to malfunction. Reuse may also create a risk of contamination of the device and lead to patient infection or cross-transfer of infection, including, but not limited to, transmission of infectious diseases and transfer of blood between patients. All this, in turn, can lead to harm to the health, illness and death of the patient.
Storage
The package of Radiesse™ Injectable Implant should be stored at controlled room temperature between 15°C and 32°C. Do not use after the expiration date. Expiration dates are indicated on product labels.
Disposal
Used and partially used syringes and injection needles may be biohazardous and should be treated and disposed of in accordance with hospital procedures and local, state or federal regulations.
Guarantee
BioForm Medical, Inc. warrants that safety precautions were used in the development and manufacture of this product.
THIS WARRANTY IS IN LIEU OF AND EXCLUDES ALL OTHER WARRANTIES NOT EXPRESSLY STATED HEREIN, WHETHER EXPRESS OR IMPLIED BY OPERATION OF LAW OR OTHERWISE, INCLUDING BUT NOT LIMITED TO ANY IMPLIED WARRANTIES OF MERCHANTABILITY QUALITY AND FITNESS FOR A PARTICULAR PURPOSE.
Storage and handling of this product, as well as factors related to the patient, diagnosis, treatment, surgical procedures and other factors beyond the control of BioForm Medical, Inc., will directly affect the product and its results. This BioForm Medical, Icl. warranty is limited to product replacement; also BioForm Medical, Inc. shall not be liable for any incidental or consequential loss, damage or expense, direct or indirect, arising from the use of this product. BioForm Medical, Inc. does not extend its liability in relation to this product to other persons or official representatives.
WHY SHOULD YOU CONTACT US
- Our clinic employs dermatologists and cosmetologists who have undergone additional training in using Radiesse and are constantly improving their skills.
- We pay great attention to consultation before the procedure, selecting rejuvenating procedures individually for each patient.
- The Beauty Medicine clinic has a cumulative discount system, so by contacting us now, you will save on subsequent procedures!
Make an appointment
by phone +7 (985) 228-44-55
via whatsapp
* There are contraindications. A doctor's consultation is required.
Carrying out the procedure
The drug is administered only in a clinical setting, although it is considered a fairly gentle method of rejuvenation. Painkillers are usually not needed. But at the request of clients, lidocaine can be applied topically - this increases the comfort of manipulation.
The rejuvenation procedure using Radiesse filler lasts no more than 20 minutes. For many women, it has become a priority way to get rid of age-related changes without plastic surgery, since it does not require recovery time.
The whole procedure consists of the following steps:
- the client sits in a special chair;
- all areas to be treated are cleaned with an antiseptic;
- marks for injections and directions of exposure are applied with a marker;
- the site for drug administration is pierced with a pointed needle, after which a blunt-tipped cannula is inserted;
- the drug is carefully injected deep under the skin in the desired direction;
- the injection site is disinfected, and the cosmetologist carefully levels the preparation, kneading areas of the face.
- similar actions are repeated in other zones to achieve symmetry;
- short-term redness caused by manual distribution of the drug in the layers of the skin quickly passes without any special measures.
This technique allows you to prolong the effect of the drug and reduce trauma. The instrument gently pushes the skin fibers apart without causing damage or subsequent hematomas.
It is also surprising that immediately after the procedure you can return to your usual rhythm of life. Sports, active recreation, sauna - there is no need to limit yourself in anything.